Abstract
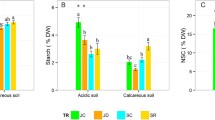
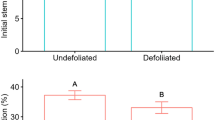
Pedunculate (Quercus robur L.) and sessile (Q. petraea [Matt.] Liebl.) oaks are the most common oak species in Western Europe. They are known to display different ecological requirements, particularly relative to root hypoxia induced by flooding: In a glasshouse study of seedlings, we quantified the effects of flooding on starch mobilization from cotyledons and starch partitioning. Growth and distribution of lateral roots were also measured. The above-ground growth of Q. robur was less affected by flooding than that of Q. petraea which failed to develop a second flush. Root growth was also severely inhibited, particularly in Q. petraea. In Q. robur, lateral root initiation as well as elongation was restricted to the soil surface layer. Flooding markedly reduced total growth and concentrations of in all components except stems. Starch mobilization from cotyledons was delayed by flooding, especially in Q. robur seedlings. Under flooding, the decrease of cotyledons dry mass and starch content in Q. robur was lower than in Q. petraea, whereas Q. robur displayed larger growth than Q. petraea. The features of carbohydrate management may be crucial in the observed differences in flooding tolerance of these species.




Similar content being viewed by others
References
Alaoui-Sossé B, Gérard B, Binet P, Toussaint M-L, Badot P-M (2005) Influence of flooding on growth, nitrogen availability in soil, and nitrate reduction of young oak seedlings (Quercus robur L.). Ann For Sci 62:593–600. doi:10.1051/forest:2005052
Alatou D, Barnola P, Lavarenne S, Gendraud M (1986) Caractérisation de la croissance rythmique du Chêne pédonculé. Plant Physiol Biochem 27:275–280
Angelov MN, Sung S-JS, Doong RL, Harms WR, Kormanik PP, Black CCJ (1996) Long- and short-term flooding effects on survival and sink-source relationships of swamp-adapted tree species. Tree Physiol 16:477–484
Bonfil C (1998) The effects of seed size, cotyledon reserves, and herbivory on seedling survival and growth in Quercus rugosa and Q. laurina (Fagaceae). Am J Bot 85:79–87. doi:10.2307/2446557
Brookes PC, Wigston DL, Bourne WF (1980) The dependence of Quercus robur and Q. petraea seedlings on cotyledon potassium, magnesium, calcium and phosphorus during the first year of growth. Forestry 53:167–177. doi:10.1093/forestry/53.2.167
Burke MK, Chambers JL (2003) Root dynamics in bottomland hardwood forests of the Southeastern United States Coastal Plain. Plant Soil 250:141–153. doi:10.1023/A:1022848303010
Colin-Belgrand M, Dreyer E, Biron P (1991) Sensitivity of seedlings from different oak species to waterlogging: effects on root growth and mineral nutrition. Ann For Sci 48:193–204. doi:10.1051/forest:19910206
Dickson RE, Tomlinson PT, Isebrands JG (2000) Partitioning of current photosynthate to different chemical fractions in leaves, stems, and roots of northern red oak seedlings during episodic growth. Can J For Res 30:1308–1317. doi:10.1139/cjfr-30-8-1308
Drew MC (1997) Oxygen deficiency and root metabolism: injury and acclimation under hypoxia and anoxia. Annu Rev Plant Physiol Plant Mol Biol 48:223–250. doi:10.1146/annurev.arplant.48.1.223
Dreyer E (1994) Compared sensitivity of seedlings from 3 woody species (Quercus robur L, Quercus rubra L and Fagus silvatica L) to water-logging and associated root hypoxia: effects on water relations and photosynthesis. Ann For Sci 51:417–429. doi:10.1051/forest:19940407
Dreyer E, Colin-Belgrand M, Biron P (1991) Photosynthesis and shoot water status of seedlings from different oak species submitted to waterlogging. Ann For Sci 48:205–214. doi:10.1051/forest:19910207
Epron D, Dreyer E (1993) Long-term effects of drought on photosynthesis of adult oak trees [Quercus petraea (Matt.) Liebl. and Quercus robur L.] in a natural stand. New Phytol 125:381–389. doi:10.1111/j.1469-8137.1993.tb03890.x
Frost I, Rydin H (1997) Effects of competition, grazing and cotyledon nutrient supply on growth of Quercus robur seedlings. Oikos 79:53–58. doi:10.2307/3546089
Fukao T, Bailey-Serres J (2004) Plant responses to hypoxia - is survival a balancing act? Trends Plant Sci 9:449–456. doi:10.1016/j.tplants.2004.07.005
García-Cebrián F, Esteso-Martínez J, Gil-Pelegrín E (2003) Influence of cotyledon removal on early seedling growth in Quercus robur L. Ann For Sci 60:69–73. doi:10.1051/forest:2002075
Gibbs J, Greenway H (2003) Mechanisms of anoxia tolerance in plants. I. Growth, survival and anaerobic catabolism. Funct Plant Biol 30:1–47. doi:10.1071/PP98095
Gravatt DA, Kirby CJ (1998) Patterns of photosynthesis and starch allocation in seedlings of four bottomland hardwood tree species subjected to flooding. Tree Physiol 18:411–417
Greenway H, Gibbs J (2003) Mechanisms of anoxia tolerance in plants. II. Energy requirements for maintenance and energy distribution to essential process. Funct Plant Biol 30:999–1036. doi:10.1071/PP98096
Guglielminetti L, Yamaguchi J, Perata P, Alpi A (1995) Amylolytic activities in cereal seeds under aerobic and anaerobic conditions. Plant Physiol 109:1069–1076
Hanson PJ, Dickson RE, Isebrands JG, Crow TR, Dixon RK (1986) A morphological index of Quercus seedling ontogeny for use in studies of physiology and growth. Tree Physiol 2:273–281
Islam MA, Macdonald SE (2004) Ecophysiological adaptations of black spruce (Picea mariana) and tamarack (Larix laricina) seedlings to flooding. Trees (Berl) 18:35–42. doi:10.1007/s00468-003-0276-9
Kabeya D, Sakai S (2003) The role of roots and cotyledons as storage organs in early stages of establishment in Quercus crispula: a quantitative analysis of the nonstructural carbohydrate in cotyledons and roots. Ann Bot (Lond) 92:537–545. doi:10.1093/aob/mcg165
Kogawara S, Yamanoshita T, Norisada M, Masumori M, Kojima K (2006) Photosynthesis and photoassimilate transport during root hypoxia in Melaleuca cajuputi, a flood-tolerant species, and in Eucalyptus camaldulensis, a moderately flood-tolerant species. Tree Physiol 26:1413–1423
Kozlowski TT (1997) Responses of woody plants to flooding and salinity
Kreuzwieser J, Papadopoulou E, Rennenberg H (2004) Interaction of flooding with carbon metabolism of forest trees. Plant Biol 6:299–306. doi:10.1055/s-2004-817882
Lévy G, Becker M, Duhamel D (1992) A comparison of the ecology of pedunculate and sessile oaks: radial growth in the centre and northwest of France. For Ecol Manage 55:51–63. doi:10.1016/0378-1127(92)90091-M
Liao CT, Lin CH (1994) Effect of flooding stress on photosynthetic activities of Mormodica charantia. Plant Physiol Biochem 32:479–485
McLeod KW, McCarron JK, Conner WH (1999) Photosynthesis and water relations of four oak species: impact of flooding and salinity. Trees (Berl) 13:178–187. doi:10.1007/s004680050231
Mielke MS, de Almeida A-AF, Gomes FP, Aguilar MAG, Mangabeira PAO (2003) Leaf gas exchange, chlorophyll fluorescence and growth responses of Genipa americana seedlings to soil flooding. Environ Exp Bot 50:221–231. doi:10.1016/S0098-8472(03)00036-4
Mustroph A, Albrecht G (2003) Tolerance of crop plants to oxygen deficiency stress: fermentative activity and photosynthetic capacity of entire seedlings under hypoxia and anoxia. Physiol Plant 117:508–520. doi:10.1034/j.1399-3054.2003.00051.x
Nguyen PV, Dickmann DI, Pregitzer KS, Hendrick R (1990) Late season changes in allocation of starch and sugar to shoot, coarse roots, and fine roots in two hybrid poplar clones. Tree Physiol 7:95–105
Parelle J, Brendel O, Bodénès C, Berveiller D, Dizengremel P, Jolivet Y, Dreyer E (2006) Differences in morphological and physiological responses to water-logging between two sympatric oak species (Quercus petraea [Matt.] Liebl., Quercus robur L.). Ann For Sci 63:849–859. doi:10.1051/forest:2006068
Perata P, Guglielminetti L, Alpi A (1997) Mobilization of endosperm reserves in cereal seeds under anoxia. Ann Bot (Lond) 79(Suppl A):49–56
Pezeshki SR (2001) Wetland plant responses to soil flooding. Environ Exp Bot 46:299–312. doi:10.1016/S0098-8472(01)00107-1
Ponton S, Dupouey J-L, Breda N, Feuillat F, Bodénès C, Dreyer E (2001) Carbon isotope discrimination and wood anatomy variations in mixed stands of Quercus robur and Quercus petraea. Plant Cell Environ 24:861–868. doi:10.1046/j.0016-8025.2001.00733.x
Schmull M, Thomas FM (2000) Morphological and physiological reactions of young deciduous trees (Quercus robur L., Q. Petraea [Matt.] Liebl., Fagus sylvatica L.) to waterlogging. Plant Soil 225:227–242. doi:10.1023/A:1026516027096
Streng DR, Glitzenstein JS, Harcombe PA (1989) Woody seedling dynamics in an east Texas floodplain forest. Ecol Monogr 59:177–204. doi:10.2307/2937285
Thomas FM (2000) Vertical rooting patterns of mature Quercus trees growing on different soil types in northern Germany. Plant Ecol 147:95–103. doi:10.1023/A:1009841921261
Thomas FM, Blank R, Hartmann G (2002) Abiotic and biotic factors and their interactions as causes of oak decline in Central Europe. For Pathol 32:277–307. doi:10.1046/j.1439-0329.2002.00291.x
Thomas FM, Hartmann G (1998) Tree rooting patterns and soil water relations of healthy and damaged stands of mature oak (Quercus robur L. and Quercus petraea [Matt.] Liebl.). Plant Soil 203:145–158. doi:10.1023/A:1004305410905
Vartapetian B (2005) Plant anaerobic stress as a novel trend in ecological physiology, biochemistry, and molecular biology: 1. Establishment of a new scientific discipline. Russ J Plant Physiol 52:826–844. doi:10.1007/s11183-005-0122-6
Vincent G, Shahriari AR, Lucot E, Badot P-M, Epron D (2006) Spatial and seasonal variations in soil respiration in a temperate deciduous forest with fluctuating water table. Soil Biol Biochem 38:2527–2535. doi:10.1016/j.soilbio.2006.03.009
Wagner PA, Dreyer E (1997) Interactive effects of waterlogging and irradiance on the photosynthetic performance of seedlings from three oak species displaying different sensitivities (Quercus robur, Q. petraea and Q. rubra). Ann For Sci 54:409–429. doi:10.1051/forest:19970501
Walls RL, Wardrop DH, Brooks RP (2005) The impact of experimental sedimentation and flooding on the growth and germinated of floodplain trees. Plant Ecol 176:203–213. doi:10.1007/s11258-004-0089-y
Yamamoto F, Sakata T, Terazawa K (1995) Physiological, morphological and anatomical responses of Fraxinus mandshurica seedlings to flooding. Tree Physiol 15:713–719
Acknowledgments
The authors are indebted to Communauté d’Agglomération du Pays de Montbéliard (CAPM) for financial support and Ph.D. fellowship for Bastien Gérard. We are grateful to the Conseil Régional de Franche-Comté and the Office National des Forêts for constant help for years. Marie-Laure Toussaint is greatly acknowledged for efficient technical support during harvests.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Adams.
Rights and permissions
About this article
Cite this article
Gérard, B., Alaoui-Sossé, B. & Badot, PM. Flooding effects on starch partitioning during early growth of two oak species. Trees 23, 373–380 (2009). https://doi.org/10.1007/s00468-008-0286-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-008-0286-8