Abstract
Purpose
Chemotherapy-induced nausea and vomiting includes both Acute (0–24 h) and Delayed (24–120 h) components with different physiologic mechanisms. A combination of a serotonin antagonist, a corticosteroid, and an NK-1 antagonist has proven effective against this problem. However, standard antiemetic regimens require administration over 3–4 days after chemotherapy. The present study evaluated a more convenient single-day three-drug antiemetic regimen for patients receiving moderately emetogenic chemotherapy.
Materials and methods
Chemotherapy-naïve patients with solid tumors receiving cyclophosphamide and/or doxorubicin were eligible. Patients could not have pre-existing etiologies for vomiting. Prior to chemotherapy, patients received a single dose of aprepitant 285 mg p.o., dexamethasone 20 mg p.o., and palonosetron 0.25 mg i.v. A daily patient diary recording episodes of emesis and severity of nausea was then kept for 5 days. Any further antiemetics were considered rescue medication.
Results
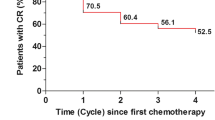
Forty-one eligible and evaluable patients (40 women, one man) with breast cancer were entered on study. Most were receiving adjuvant chemotherapy. Complete Response (no vomiting, no rescue medication) was seen in 51% of patients, including 76% with Complete Response for the Acute period and 66% for the Delayed period. No emesis was reported for 100% of patients in the Acute period and 95% in the Delayed period. No Nausea was seen in 32% of patients. No untoward toxicities were seen.
Conclusion
A single-day three-drug antiemetic regimen is feasible and effective for protection against both Acute and Delayed vomiting after moderately emetogenic chemotherapy. Formal comparison to a standard multi-day antiemetic regimen is warranted.


Similar content being viewed by others
References
ASHP (1999) ASHP therapeutic guidelines on the pharmacologic management of nausea and vomiting in adult and pediatric patients receiving chemotherapy or radiation therapy or undergoing surgery. Am J Health Syst Pharm 56:729–764
Beck TM, Hesketh PH, Madajewicz S et al (1992) Stratified, randomized, double-blind comparison of intravenous ondansetron administered as a multiple-dose regimen versus two single-dose regimens in the prevention of cisplatin-induced nausea and vomiting. J Clin Oncol 10:1969–1975
Blum RA, Majumdar A, McCrea J et al (2003) Effects of aprepitant on the pharmacokinetics of ondansetron and granisetron in healthy subjects. Clin Ther 25:1407–1419 doi:10.1016/S0149-2918(03)80128-5
Chawla SP, Grunberg SM, Gralla RJ et al (2003) Establishing the dose of the oral NK1 antagonist aprepitant for the prevention of chemotherapy-induced nausea and vomiting. Cancer 97:2290–2300 doi:10.1002/cncr.11320
Cocquyt V, Van Belle S, Reinhardt RR et al (2001) Comparison of L-758,298, a prodrug for the selective neurokinin-1 antagonist, L-754,030, with ondansetron for the prevention of cisplatin-induced emesis. Eur J Cancer 37:835–842 doi:10.1016/S0959-8049(00)00416-0
Cohen L, de Moor CA, Eisenberg P et al (2007) Chemotherapy-induced nausea and vomiting—incidence and impact on patient quality of life at community oncology settings. Support Care Cancer 15:497–503 doi:10.1007/s00520-006-0173-z
De Jongh Garcia C, Poli S, Ananth C et al (2005) Health care provider perception of nausea and vomiting and patients’ reported incidence: the Venezuela Emesis Registry. Support Care Cancer 13:414–415 (abstr 04-022)
De Wit R (2003) Current position of 5HT3 antagonists and the additional value of NK1 antagonists; a new class of antiemetics. Br J Cancer 88:182–187 doi:10.1038/sj.bjc.6601033
Eisenberg P, Figueroa-Vadillo J, Zamora R et al (2003) Improved prevention of moderately emetogenic chemotherapy-induced nausea and vomiting with palonosetron, a pharmacologically novel 5-HT3 receptor antagonist: results of a phase III, single-dose trial versus dolasetron. Cancer 98:1473–1482 doi:10.1002/cncr.11817
Erazo Valle A, Wisniewski T, Figueroa Vadillo JI et al (2006) Incidence of chemotherapy-induced nausea and vomiting in Mexico: healthcare provider predictions versus observed. Curr Med Res Opin 22:2403–2410 doi:10.1185/030079906X154033
Ettinger DS, Bierman PJ, Bradbury B et al (2007) Antiemesis. J Natl Compr Canc Netw 5:12–33
Gralla R, Lichinitser M, Van Der Vegt S et al (2003) Palonosetron improves prevention of chemotherapy-induced nausea and vomiting following moderately emetogenic chemotherapy: results of a double-blind randomized phase III trial comparing single doses of palonosetron with ondansetron. Ann Oncol 14:1570–1577 doi:10.1093/annonc/mdg417
Griffin AM, Butow PN, Coates AS et al (1996) On the receiving end. V: Patient perceptions of the side effects of cancer chemotherapy in 1993. Ann Oncol 7:189–195
Grote T, Hajdenberg J, Cartmell A et al (2006) Combination therapy for chemotherapy-induced nausea and vomiting in patients receiving moderately emetogenic chemotherapy: palonosetron, dexamethasone, and aprepitant. J Support Oncol 4:403–408
Grunberg SM, Deuson RR, Mavros P et al (2004) Incidence of chemotherapy-induced nausea and emesis after modern antiemetics. Cancer 100:2261–2268 doi:10.1002/cncr.20230
Grunberg S, Ettinger DS, Hauber AB et al (2008) How familiar are oncologists with therapeutic care and supportive care guidelines? Support Care Cancer 16:631–632 (abstr 01-007)
Herrington JD, Jaskiewicz AD, Song J (2008) Randomized, placebo-controlled, pilot study evaluating aprepitant single dose plus palonosetron and dexamethasone for the prevention of acute and delayed chemotherapy-induced nausea and vomiting. Cancer 112:2080–2087 doi:10.1002/cncr.23364
Hesketh PJ, Grunberg SM, Gralla RJ et al (2003) The oral neurokinin-1 antagonist aprepitant for the prevention of chemotherapy-induced nausea and vomiting: a multinational, randomized, double-blind, placebo-controlled trial in patients receiving high-dose cisplatin—the Aprepitant Protocol 052 Study Group. J Clin Oncol 21:4112–4119 doi:10.1200/JCO.2003.01.095
Horgan KJ, Petty K, Majumdar A et al (2003) Treatment options for chemotherapy-induced nausea and vomiting: current and future. Am J Cancer 2:391–392 doi:10.2165/00024669-200302050-00014
Kripalani S, Price M, Vigil V et al (2008) Frequency and predictors of prescription-related issues after hospital discharge. J Hosp Med 3:12–19 doi:10.1002/jhm.248
Kris MG, Gralla RJ, Clark RA et al (1988) Dose-ranging evaluation of the serotonin antagonist GR-C507/75 (GR38032F) when used as an antiemetic in patients receiving anticancer chemotherapy. J Clin Oncol 6:659–662
Kris MG, Hesketh PJ, Somerfield MR et al (2006) American Society of Clinical Oncology guideline for antiemetics in oncology: update 2006. J Clin Oncol 24:2932–2947 doi:10.1200/JCO.2006.06.9591
Liau CT, Chiu NM, Liu HE et al (2005) Incidence of chemotherapy-induced nausea and vomiting in Taiwan: physicians’ and nurses’ estimation vs. patients’ reported outcomes. Support Care Cancer 13:277–286 doi:10.1007/s00520-005-0788-5
Loos WJ, de Wit R, Freedman SJ et al (2007) Aprepitant when added to a standard antiemetic regimen consisting of ondansetron and dexamethasone does not affect vinorelbine pharmacokinetics in cancer patients. Cancer Chemother Pharmacol 59:407–412 doi:10.1007/s00280-006-0359-6
Maranzano E, Feyer PC, Molassiotis A et al (2005) Evidence-based recommendations for the use of antiemetics in radiotherapy. Radiother Oncol 76:227–233 doi:10.1016/j.radonc.2005.07.002
McCrea JB, Majumdar AK, Goldberg MR et al (2003) Effects of the neurokinin-1 receptor antagonist aprepitant on the pharmacokinetics of dexamethasone and methylprednisolone. Clin Pharmacol Ther 74:17–24 doi:10.1016/S0009-9236(03)00066-3
Modi AC, Morita DA, Glauser TA (2008) One-month adherence in children with new-onset epilepsy: white-coat compliance does not occur. Pediatrics 121:e961–e966 doi:10.1542/peds.2007-1690
Navari RM, Reinhardt RR, Gralla RJ et al (1999) Reduction of cisplatin-induced emesis by a selective neurokinin-1-receptor antagonist. L-754,030 Antiemetic Trials Group. N Engl J Med 340:190–195 doi:10.1056/NEJM199901213400304
Nygren P, Hande K, Petty KJ et al (2005) Lack of effect of aprepitant on the pharmacokinetics of docetaxel in cancer patients. Cancer Chemother Pharmacol 55:609–616 doi:10.1007/s00280-004-0946-3
Partridge AH, LaFountain A, Mayer E et al (2008) Adherence to initial adjuvant anastrozole therapy among women with early-stage breast cancer. J Clin Oncol 26:556–562 doi:10.1200/JCO.2007.11.5451
Poli-Bigelli S, Rodrigues-Pereira J, Carides AD et al (2003) Addition of the neurokinin 1 receptor antagonist aprepitant to standard antiemetic therapy improves control of chemotherapy-induced nausea and vomiting. Results from a randomized, double-blind, placebo-controlled trial in Latin America. Cancer 97:3090–3098 doi:10.1002/cncr.11433
Sandoval C, Corbi D, Strobino B et al (1999) Randomized double-blind comparison of single high-dose ondansetron and multiple standard-dose ondansetron in chemotherapy-naïve pediatric oncology patients. Cancer Invest 17:309–313
Shah AK, Hunt TL, Gallagher SC et al (2005) Pharmacokinetics of palonosetron in combination with aprepitant in healthy volunteers. Curr Med Res Opin 21:595–601 doi:10.1185/030079905X40481
Simon R (1989) Optimal two-stage designs for phase II clinical trials. Control Clin Trials 10:1–10 doi:10.1016/0197-2456(89)90015-9
Warr DG, Hesketh PJ, Gralla RJ et al (2005) Efficacy and tolerability of aprepitant for the prevention of chemotherapy-induced nausea and vomiting in patients with breast cancer after moderately emetogenic chemotherapy. J Clin Oncol 23:2822–2830 doi:10.1200/JCO.2005.09.050
Wu JR, Moser DK, Lennie TA et al (2008) Medication adherence in patients who have heart failure: a review of the literature. Nurs Clin North Am 43:133–153 doi:10.1016/j.cnur.2007.10.006
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported in part by Grant Number P30CA022435 from the National Cancer Institute and by an unrestricted grant from MGI Pharma.
Dr. Grunberg has served as a consultant to MGI Pharma and Merck.
Rights and permissions
About this article
Cite this article
Grunberg, S.M., Dugan, M., Muss, H. et al. Effectiveness of a single-day three-drug regimen of dexamethasone, palonosetron, and aprepitant for the prevention of acute and delayed nausea and vomiting caused by moderately emetogenic chemotherapy. Support Care Cancer 17, 589–594 (2009). https://doi.org/10.1007/s00520-008-0535-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-008-0535-9