Abstract
Goals
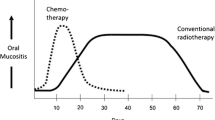
Oral mucositis can be a significant and dose-limiting complication of high-dose cancer therapy. Mucositis is a particularly severe problem in patients receiving myeloablative chemotherapy prior to bone marrow or hematopoetic stem cell transplant (HSCT). The cyclooxygenase (COX) pathway mediates tissue injury and pain through upregulation of pro-inflammatory prostaglandins, including prostaglandin E2 (PGE2) and prostacyclin (PGI2). The objective of this small (n = 3) pilot study was to examine the role of the COX pathway in causing mucosal injury and pain in chemotherapy-induced oral mucositis.
Materials and methods
We collected blood, saliva, and oral mucosal biopsy specimens from three autologous HSCT patients at the following time-points before and after administration of conditioning chemotherapy: Day −10, +10, +28, and +100, where day 0 is day of transplant. RNA extracted from full-thickness tissue samples was measured by RT-PCR for the following: COX-1, COX-2, microsomal prostaglandin E synthase (mPGES), IL-1β, and TNF-α. Blood and saliva samples were measured by ELISA for PGE2 and PGI2, which are markers of COX activity. Severity of oral mucositis was determined using the Oral Mucositis Index. Severity of pain due to oral mucositis was measured using a Visual Analog Scale. Relationships between the different variables were examined using Spearman rank correlation coefficients.
Main results
Mean mucositis and pain scores increased significantly after administration of chemotherapy and then gradually declined. The correlation between changes in mucositis and pain scores was strong and statistically significant. The following additional correlations were statistically significant: between tissue COX-1 and pain; between tissue mPGES and pain; between salivary PGE1 and pain; between salivary PGI2 and pain. Other relationships were not statistically significant.
Conclusions
Our finding of significant associations of pain scores with tissue COX-1 and mPGES, as well as salivary prostaglandins, is suggestive of a role for the cyclooxygenase pathway in mucositis, possibly via upregulation of pro-inflammatory prostaglandins. However, our small sample size may have contributed to the lack of significant associations between COX-2 and other inflammatory mediators with mucosal injury and pain. Thus, additional studies with larger numbers of subjects are warranted to confirm the involvement of the cyclooxygenase pathway in chemotherapy-induced mucositis.








Similar content being viewed by others
References
6-keto Prostaglandin F1a EIA Kit. [Internet document] Available at: http://www.caymanchem.com/app/template/Product.vm/catalog/515211/a/z. Accessed 11 February, 2009
Bellm LA, Epstein JB, Rose-Ped A, Martin P, Fuchs HJ (2000) Patient reports of complications of bone marrow transplantation. Support Care Cancer 8:33–39
Cheng KK (2007) Oral mucositis and quality of life of Hong Kong Chinese patients with cancer therapy. Eur J Oncol Nurs 11:36–42
Choi SH, Langenbach R, Bosetti F (2008) Genetic deletion or pharmacological inhibition of cyclooxygenase-1 attenuate lipopolysaccharide-induced inflammatory response and brain injury. Faseb J 22:1491–1501
Diaz A, Chepenik KP, Korn JH, Reginato AM, Jimenez SA (1998) Differential regulation of cyclooxygenases 1 and 2 by interleukin-1 beta, tumor necrosis factor-alpha, and transforming growth factor-beta 1 in human lung fibroblasts. Exp Cell Res 241:222–229
Doi Y, Minami T, Nishizawa M, Mabuchi T, Mori H, Ito S (2002) Central nociceptive role of prostacyclin (IP) receptor induced by peripheral inflammation. Neuroreport 13:93–96
Elting LS, Cooksley C, Chambers M, Cantor SB, Manzullo E, Rubenstein EB (2003) The burdens of cancer therapy. Clinical and economic outcomes of chemotherapy-induced mucositis. Cancer 98:1531–1539
Epstein JB, Schubert MM (2004) Managing pain in mucositis. Semin Oncol Nurs 20:30–37
Feng CJ, Guo JB, Jiang HW et al (2008) Spatio-temporal localization of HIF-1alpha and COX-2 during irradiation-induced oral mucositis in a rat model system. Int J Radiat Biol 84:35–45
Hong JH, Chiang CS, Tsao CY, Lin PY, McBride WH, Wu CJ (1999) Rapid induction of cytokine gene expression in the lung after single and fractionated doses of radiation. Int J Radiat Biol 75:1421–1427
Huskisson EC (1974) Measurement of pain. Lancet 2:1127–1131
Lalla RV, Peterson DE (2005) Oral mucositis. Dent Clin North Am 49:167–184
Lalla RV, Peterson DE (2006) Treatment of mucositis, including new medications. Cancer J 12:348–354
Lalla RV, Sonis ST, Peterson DE (2008) Management of oral mucositis in patients who have cancer. Dent Clin North Am 52:61–77
Lin CR, Amaya F, Barrett L et al (2006) Prostaglandin E2 receptor EP4 contributes to inflammatory pain hypersensitivity. J Pharmacol Exp Ther 319:1096–1103
Logan RM, Gibson RJ, Sonis ST, Keefe DM (2007) Nuclear factor-kappaB (NF-kappaB) and cyclooxygenase-2 (COX-2) expression in the oral mucosa following cancer chemotherapy. Oral Oncol 43:395–401
McGuire DB, Peterson DE, Muller S, Owen DC, Slemmons MF, Schubert MM (2002) The 20 item oral mucositis index: reliability and validity in bone marrow and stem cell transplant patients. Cancer Invest 20:893–903
Prostaglandin E Metabolite EIA kit. [Internet document] Available at: http://www.caymanchem.com/app/template/Product.vm/catalog/514531/a/z. Accessed 11 February, 2009
Rapoport AP, Miller Watelet LF, Linder T et al (1999) Analysis of factors that correlate with mucositis in recipients of autologous and allogeneic stem-cell transplants. J Clin Oncol 17:2446–2453
Rouzer CA, Marnett LJ (2008) Cyclooxygenases: structural and functional insights. J Lipid Res; 50: S29-34S. April 2009
Ruescher TJ, Sodeifi A, Scrivani SJ, Kaban LB, Sonis ST (1998) The impact of mucositis on alpha-hemolytic streptococcal infection in patients undergoing autologous bone marrow transplantation for hematologic malignancies. Cancer 82:2275–2281
Schubert MM, Sullivan KM, Morton TH et al (1984) Oral manifestations of chronic graft-v-host disease. Arch Intern Med 144:1591–1595
Schubert MM, Williams BE, Lloid ME, Donaldson G, Chapko MK (1992) Clinical assessment scale for the rating of oral mucosal changes associated with bone marrow transplantation. Development of an oral mucositis index. Cancer 69:2469–2477
Shankavaram UT, Lai WC, Netzel-Arnett S et al (2001) Monocyte membrane type 1-matrix metalloproteinase. Prostaglandin-dependent regulation and role in metalloproteinase-2 activation. J Biol Chem 276:19027–19032
Sonis ST, Peterson RL, Edwards LJ et al (2000) Defining mechanisms of action of interleukin-11 on the progression of radiation-induced oral mucositis in hamsters. Oral Oncol 36:373–381
Sonis ST, Oster G, Fuchs H et al (2001) Oral mucositis and the clinical and economic outcomes of hematopoietic stem-cell transplantation. J Clin Oncol 19:2201–2205
Sonis ST, O'Donnell KE, Popat R et al (2004) The relationship between mucosal cyclooxygenase-2 (COX-2) expression and experimental radiation-induced mucositis. Oral Oncol 40:170–176
Spielberger R, Stiff P, Bensinger W et al (2004) Palifermin for oral mucositis after intensive therapy for hematologic cancers. N Engl J Med 351:2590–2598
Tanner NS, Stamford IF, Bennett A (1981) Plasma prostaglandins in mucositis due to radiotherapy and chemotherapy for head and neck cancer. Br J Cancer 43:767–771
Xun CQ, Thompson JS, Jennings CD, Brown SA, Widmer MB (1994) Effect of total body irradiation, busulfan-cyclophosphamide, or cyclophosphamide conditioning on inflammatory cytokine release and development of acute and chronic graft-versus-host disease in H-2- incompatible transplanted SCID mice. Blood 83:2360–2367
Zidar N, Odar K, Glavac D, Jerse M, Zupanc T, Stajer D (2009) Cyclooxygenase in normal human tissues - is COX-1 really a constitutive isoform, and COX-2 an inducible isoform? J Cell Mol Med; July 24 (Epub ahead of print)
Acknowledgments
Dr. Lalla’s effort on this research was supported by grants T32DE007302 and K23DE016946 from the NIH. This research was supported in part by a General Clinical Research Center (GCRC) grant (M01RR06192) from the NIH awarded to the University of Connecticut Health Center. We thank Ms. Pamela Fall, Ms. Christine Abreu and Dr. Jonathan Covault of the GCRC Core Laboratory for implementation of the quantitative RT-PCR and ELISA analyses. We thank Ms. Kim Jennings of the GCRC Clinical Core for study coordination and data entry.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lalla, R.V., Pilbeam, C.C., Walsh, S.J. et al. Role of the cyclooxygenase pathway in chemotherapy-induced oral mucositis: a pilot study. Support Care Cancer 18, 95–103 (2010). https://doi.org/10.1007/s00520-009-0635-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-009-0635-1