Abstract
Purpose
The clinical sedation scores available for assessing sedation in the intensive care unit (ICU) have drawbacks and limit their usefulness in paralyzed and deeply sedated patients. An objective tool, the bispectral index (BIS), could prove beneficial in such circumstances. We evaluated the ability of BIS to assess the level of sedation and its correlation with the Richmond agitation sedation scale (RASS) in ICU.
Methods
Twenty-four, mechanically ventilated, critically ill patients of either sex, 15–65 years of age, were studied over a period of 24 h. They received a standard sedation regimen consisting of a bolus dose of propofol 0.5 mg/kg and fentanyl 1 μg/kg followed by infusions of propofol and fentanyl ranging from 1.5 to 5 mg/kg/h and 0.5 to 2.0 μg/kg/h, respectively. Hemodynamic parameters, temperature, end-tidal carbon dioxide, BIS and RASS values were recorded. The correlation of BIS and RASS was expressed as Kendall correlation coefficients (τ). A p value of <0.05 was considered statistically significant.
Results
A total of 414 readings was obtained. On comparing BIS values for all patients with the corresponding RASS values, there was a statistically highly significant correlation between the two. (τ = 0.56, p < 0.0001). For adequate sedation as judged by a RASS value of 0 to −3, the median BIS value was found to be 56 (range 42–89). A BIS value of 70 had a high sensitivity (85%) and specificity (80%) to differentiate adequate from inadequate sedation.
Conclusion
Our results illustrate that BIS correlates well with RASS when assessing the level of sedation in mechanically ventilated critically ill patients. BIS reliably differentiates inadequate from adequate sedation.
Similar content being viewed by others
Introduction
Sedation and analgesia is an important part of therapy for patients in intensive care units (ICU). It reduces anxiety and stress, facilitates sleep, prevents injuries and accidental removal of catheters, and reduces resistance to mechanical ventilation [1]. It has been demonstrated that optimizing sedation in intensive care patients reduces the period of mechanical ventilation, ICU stay, and hospital stay [2]. Adequate sedation can be ensured by monitoring the level of sedation using various clinical sedation scores [3]. However, the inter-rater variability, difficulty to obtain reproducibility, and discomfort caused by these methods are major hindrances in their use [4, 5]. In addition, these cannot be applied to patients receiving neuromuscular blocking agents and those under deep sedation, necessitating the need for objective tools of assessing sedation [6].
A newer sedation score called the Richmond agitation sedation scale (RASS) [7] has been introduced into practice that has a distinct advantage in discretely separating verbal from physical stimulation and is one of the few sedation scores that has been appropriately tested for reliability and validity [4, 8]. Bispectral index (BIS) monitoring provides a continuous and observer-independent digital value representing cerebral activity and is one of the few available objective tools to monitor the level of sedation. Several studies have examined the utility of BIS to guide sedation in mechanically ventilated patients in the medical and surgical ICU but have shown varying results [9]. Previous studies attempted to evaluate the correlation of BIS and RASS in ICU patients but either did not use a standard protocol of sedation [8] or included neurologically impaired patients [10]. In this study, we evaluated both the utility of BIS to assess the level of sedation and its correlation with the RASS in critically ill patients using a standard protocol of sedation with propofol and fentanyl. We hypothesized that BIS monitoring would allow differentiation among clinically relevant levels of sedation and, hence, be of potential benefit when clinical examination was not possible.
Materials and methods
This prospective, comparative, single-blinded observer study was conducted in the ICU, Department of Anaesthesiology and Intensive Care, All India Institute of Medical Sciences, after approval by the tnstitute ethics committee. A written, informed consent was obtained from patients’ relatives. Twenty-four critically ill patients of either sex, 16–60 years of age, admitted to the ICU were included. Patients with neurological impairment, hemodynamic instability, hypothermia (core temperature <36°C), or receiving neuromuscular blockade or sedative drugs other than the prescribed regimen were excluded from the study. All patients were monitored with continuous electrocardiogram (ECG), invasive/noninvasive blood pressure, pulse oximetry, capnography, temperature, and BIS. After inclusion in the study, they received a standard sedation regimen consisting of a bolus dose of propofol 0.5 mg/kg and fentanyl 1 μg/kg followed by infusions of propofol and fentanyl ranging from 1.5 to 5 mg/kg/h and 0.5 to 2.0 μg/kg/h, respectively. The infusions were titrated with the aim of keeping the RASS score between 0 and −3. Adequate sedation was achieved by administration of a bolus of propofol 0.5 mg/kg followed by an increase in the infusion rate by 0.5 mg/kg per hour. Similarly, a bolus of fentanyl 0.5 μg/kg was followed by an increase in the infusion rate by 0.5 μg/kg/h every half hour till the patient achieved an RASS score between 0 and −3.
BIS electrodes were placed above the bridge of the nose, over the temple area, and between the medial corner of the eye and the hairline after the areas had been adequately cleaned with spirit as per the manufacturer’s instructions. Electrodes were repositioned when impedance increased significantly enough to impair the electroencephalogram (EEG) evaluation. BIS values were measured using the BIS monitor (model A-2000, software version 3.21; Aspect Medical Systems Inc, Newton, MA, USA). Before measuring BIS values, signal quality index (SQI) >80% was ensured. Electromyography (EMG) values were also recorded for assessing their influence on BIS scores. BIS values were recorded every 5 s for 15 s, and the mean value of the three readings was taken as the final value. The coinvestigator took the RASS reading (Table 1) after BIS values had been recorded and the BIS monitor covered.
Simultaneous recording of RASS and BIS values, along with the other variables, were performed first on admission to ICU, every 15 min for the first 2 h, and then every 2 h for 24 h. Care was taken to avoid taking readings within half an hour of any stimulation; endotracheal tube suctioning, physiotherapy or any other ICU procedures. To evaluate whether BIS could distinguish among more clinically relevant levels of sedation, RASS was divided into three groups: inadequate (RASS +4 to +1), adequate (RASS 0 to −3), and excessive (RASS −4 and −5).
Data analysis
Data were analyzed using SPSS version 11.0 software (SPSS Inc., Chicago, IL, USA). Central tendencies were described with the mean and median values. Dispersion was described with the standard deviation (SD) and interquartile range, whichever was applicable. Correlation of BIS and the ordinal-scaled RASS was expressed as Kendall correlation coefficient (τ). A p value of <0.05 was considered statistically significant. Sensitivity and specificity of various BIS values were calculated using receiver operating characteristic (ROC) curves to determine the ability of BIS to differentiate between adequate and inadequate sedation.
Results
Twenty-four patients were included in the study, and 414 observations were obtained. Fourteen patients were men and the rest were women. Mean age was 39 ± 14 years and mean weight 58.3 ± 10.6 kg. The majority (19 patients) were postoperative patients, whereas the rest suffered from a medical disorder. Four patients had to be excluded from the study after a few hours: two patients required paralysis for institution of inverse ratio ventilation, and the other two developed dysrhythmias and hypotension, necessitating discontinuation of propofol. The readings of these patients to the time they were paralyzed or sedation was discontinued were included for statistical analysis.
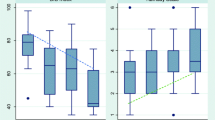
With increasing levels of sedation as measured by RASS, a decrease in BIS values was seen (Fig. 1). On comparing BIS values for all patients at all times with the corresponding RASS values, there was a statistically highly significant correlation between the two (τ = 0.56, p < 0.0001). Of the 414 BIS readings, 29 were associated with excessive EMG interference (>50%). A subset analysis of the remainder of the filtered data yielded only a slightly better correlation between BIS and RASS (τ: 0.57 vs. 0.56). Median and interquartile range of BIS values observed for each level of RASS is depicted in Fig. 1. For adequate sedation as judged by an RASS value of 0 to −3, the median BIS value was 56 (range 42–89). A BIS value of 70 was had a sensitivity of 85% and specificity of 80% for differentiating adequate (RASS 0 to −3) from inadequate (RASS +4 to +1) sedation.
Box plot showing distribution of bispectral index (BIS) for each level of Richmond agitation sedation scale (RASS). Boxes represent the interquartile range [25th percentile (lower edge) and 75th percentile (upper edge)]. The median BIS is denoted as the line across the box. Upper and lower lines extend from the corresponding percentile to the highest and lowest values, excluding outliers. Circles represent outliers (cases with values between 1.5 and 3 box lengths from the upper or lower edge in the box). Asterisks represent extremes (cases with >3 box lengths)
Discussion
Intensive care therapy often requires patient sedation to avoid stress caused by underlying disease processes, pain, or altered perception of the environment [11]. However, optimizing sedation is crucial, as both over- and under-sedation are fraught with serious consequences. The use of subjective scoring systems to assess sedation in this patient population is strongly recommended because it promotes optimal patient-focused sedation management [12]. RASS was found in a recent survey with excellent inter-rater reliability and validity to be one of the most frequently used sedation scales [13]. However, it may be found wanting in cases of very deep sedation and patients with neuromuscular blockade [6]. Use of an objective method to monitor sedation, such as BIS, in such situations can lead to a more appropriate and judicious use of sedative medications. We performed this study to evaluate the usefulness of BIS in assessing sedation in the ICU and its correlation with the RASS.
We found a highly significant (p < 0.001) correlation between BIS and RASS values (τ = 0.562) using a standardized protocol of sedation. Deogaonkar and colleagues [10] reported a statistically significant correlation between RASS and BIS (R 2 = 0.471, p < 0.001) in ICU patients. However, their patients were neurologically impaired, and only some received sedative medications, the details of which are not mentioned. Another study assessing the correlation between RASS and BIS in patients admitted to the medical ICU had similar findings, but the sedation regime was not standardized, and the patients received multiple drugs such as morphine, fentanyl, midazolam, and lorazepam [8]. In a recent study by Turkmen et al. [14], RASS and BIS were compared in eleven mechanically ventilated critically ill patients sedated with dexmedetomidine, and a statistically significant correlation was found between the two (r = 0.9; p = 0.0001). As the number of patients as well as observation duration was significantly longer in our study, it further strengthens the association between the two.
Our results show that BIS monitoring can be easily applied in the ICU setting, even though the conditions are less than ideal, with the presence of many possible sources of artifacts. The problem may be compounded in lightly sedated patients in whom excessive myogenic activity can increase the artifacts, causing difficulty in interpretation of the signal [15, 16]. In our study, only 29 BIS readings had excessive EMG interference, the reason for which is not entirely clear. It could be possible that the fentanyl infusion provided potent analgesia and therefore there was less EMG interference in our patients [17]. In fact, BIS changes accompanying neuromuscular blockade have raised issues about the adequacy of sedation and analgesia administered to ICU patients [18].
We used a relatively newer clinical sedation score, the RASS, in our study, which is often confused with Riker’s sedation agitation scale (SAS). The SAS is a single-item 7-point scale developed by Riker [19], with scores ranging from a minimum value of 1 (deep sedation) to a maximum value of 7 (severe agitation). RASS is also a single-item numerical structure but has 10 levels of response that range from −4 to +5, thus avoiding the complexity of summing multiple subscale scores [4]. Though sedation and agitation are evaluated in a single scale, the sequential approach helps establish a single score by first assessing agitation and then assessing sedation. RASS appears to be a more appropriate score for mechanically ventilated patients, as they generally need light to moderate sedation [2, 20], and it offers multiple levels (0 to −3) within this range. RASS offers broader discrimination in the commonly used mild to moderate sedation range in the ICU than other commonly used scales [4]. Similarly, RASS was also found to have high inter-rater reliability for virtually all categories of adult ICU patients [8].
Although clinical sedation scales use a varying number of actual scores, their most clinically relevant function is differentiation among inadequate, optimal, or excessive sedation [21].Therefore, comparison of BIS with these scales is dependent on the criteria used to define these categories. A RASS score of 0 to −3 was found to be associated with light to moderate sedation, which is desired in most of patients admitted to the ICU. A score more than that would indicate inadequate sedation and a score of −4 or −5 would indicate excessive sedation.
The BIS value of 70 is recommended for ensuring an adequate level of sedation but may have greater probability of consciousness and a potential for recall [22]. Therefore, setting a target value of 70 for sedation in critically ill patients could possibly avoid the complications associated with inadequate sedation. To differentiate adequate from inadequate sedation in our study, a BIS value of 70 had a sensitivity of 85% and a specificity of 80%. This is consistent with the findings of Berkenbosch et al. [21], who found BIS value <70 has a sensitivity of 87% to differentiate adequate from inadequate sedation. For adequate sedation as judged by an RASS value of 0 to −3, the median BIS value was found to be 56 and values ranged from 42 to 89. This is in accordance with the study by Ely et al. in which an RASS score of −1 to −3 was seen to correspond to a median BIS value of 69.0 (57.6–87.6).
We did not compare BIS with sedation scales other than RASS, which would have further given credibility to our findings. We were limited in our ability to assess the utility of BIS in distinguishing between excessive and adequate levels of sedation, as we had no patients with RASS scores of −4 and −5. We found that recording regular BIS readings is difficult for long durations, especially in critically ill patients. The presence of edema, sweating, and excessive movement during suctioning, physiotherapy, or any other procedure dislodges the electrode. The need for better electrodes with longer-lasting and resistant adhesives needs to be emphasized. Further studies focused on the contributions and impact of BIS on patient outcome are needed. Despite these limitations, our results illustrate that BIS monitoring, an objective method of assessing sedation, could prove useful in continuously assessing the level of sedation in mechanically ventilated critically ill patients.
References
Riess ML, Graefe UA, Goeters C, Van Aken H, Bone HG. Sedation assessment in critically ill patients with bispectral index. Eur J Anaesthesiol. 2002;19:18–22.
Kress JP, Pohlman AS, O’Connor MF, Hall JB. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med. 2000;342:1471–7.
De Jonghe B, Cook D, Appere-De-Vecchi C, Guyatt G, Meade M, Outin H. Using and understanding sedation scoring systems: a systematic review. Intensive Care Med. 2000;26:275–85.
Sessler CN, Gosnell MS, Grap MJ, Brophy GM, O’Neal PV, Keane KA, Tesoro EP, Elswick RK. The Richmond sedation–agitation scale: validity and reliability in adult intensive care patients. Am J Respir Crit Care Med. 2002;166:1338–44.
Riker RR, Fraser GL, Simmons LE, Wilkins ML. Validating the sedation agitation scale with the bispectral index and visual analog scale in adult ICU patients after cardiac surgery. Intensive Care Med. 2001;27:853–8.
Ramsay M. Role of brain function monitoring in the critical care and perioperative settings. Semin Anesth Periop Med Pain. 2005;24:195–202.
Sessler CN, Grap MJ, Brophy GM. Multidisciplinary management of sedation and analgesia in critical care. Semin Respir Crit Care Med. 2001;22:211–25.
Ely EW, Truman B, Shintani A, Thomason JW, Wheeler AP, Gordon S, Francis J, Speroff T, Gautam S, Margolin R, Sessler CN, Dittus RS, Bernard GR. Monitoring sedation status over time in ICU patients: reliability and validity of the Richmond agitation–sedation scale. JAMA. 2003;289:2983–91.
Olson DM, Thoyre SM, Auyong DB. Perspectives on sedation assessment in critical care. AACN Adv Crit Care. 2007;18:380–95.
Deogaonkar A, Gupta R, DeGeorgia M, Sabharwal V, Gopakumaran B, Schubert A, Provencio JJ. Bispectral index monitoring correlates with sedation scales in brain-injured patients. Crit Care Med. 2004;32:2403–6.
Tonner PH, Wei C, Bein B, Weiler N, Paris A, Scholz J. Comparison of two bispectral index algorithms in monitoring sedation in postoperative intensive care patients. Crit Care Med. 2005;33:580–4.
Sessler CN, Grap MJ, Ramsay MA. Evaluating and monitoring analgesia and sedation in the intensive care unit. Crit Care. 2008;12(Suppl 3):S2.
Payen JF, Chanques G, Mantz J, Hercule C, Auriant I, Leguillou JL, Binhas M, Genty C, Rolland C, Bosson JL. Current practices in sedation and analgesia for mechanically ventilated critically ill patients: a prospective multicenter patient-based study. Anesthesiology. 2007;106:687–95.
Turkmen A, Altan A, Turgut N, Vatansever S, Gokkaya S. The correlation between the Richmond agitation–sedation scale and bispectral index during dexmedetomidine sedation. Eur J Anaesthesiol. 2006;23:300–4.
Vivien B, Di Maria S, Ouattara A, Langeron O, Coriat P, Riou B. Overestimation of bispectral index in sedated intensive care unit patients revealed by administration of muscle relaxant. Anesthesiology. 2003;99:9–17.
Nasraway SA Jr, Wu EC, Kelleher RM, Yasuda CM, Donnelly AM. How reliable is the bispectral index in critically ill patients. A prospective, comparative, single-blinded observer study. Crit Care Med. 2002;30:1483–7.
Lu C, Man K, Ou-Yang H, Chan S, Ho S, Wong C, Liaw W. Composite auditory evoked potential index versus bispectral index to estimate the level of sedation in paralyzed critically ill patients: a prospective observational study. Anesth Analg. 2008;107:1290–4.
Dahaba AA, Mattweber M, Fuchs A, Zenz W, Rehak PH, List WF, Metzler H. The effect of different stages of neuromuscular block on the bispectral index and the bispectral index-XP under remifentanil/propofol anesthesia. Anesth Analg. 2004;99:781–7.
Riker RR, Fraser GL, Cox PM. Continuous infusion of haloperidol controls agitation in critically ill patients. Crit Care Med. 1994;22:433–40.
Brook AD, Ahrens TS, Schaiff R, Prentice D, Sherman G, Shannon W, Kollef MH. Effect of a nursing implemented sedation protocol on the duration of mechanical ventilation. Crit Care Med. 1999;27:2609–15.
Berkenbosch JW, Fichter CR, Tobias JD. The correlation of the bispectral index monitor with clinical sedation scores during mechanical ventilation in the pediatric intensive care unit. Anesth Analg. 2002;94:506–11.
Liu SS. Effects of bispectral index monitoring on ambulatory anesthesia: a meta-analysis of randomized controlled trials and a cost analysis. Anesthesiology. 2004;101:311–5.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Karamchandani, K., Rewari, V., Trikha, A. et al. Bispectral index correlates well with Richmond agitation sedation scale in mechanically ventilated critically ill patients. J Anesth 24, 394–398 (2010). https://doi.org/10.1007/s00540-010-0915-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00540-010-0915-4