Abstract
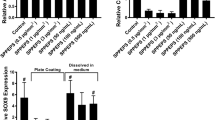
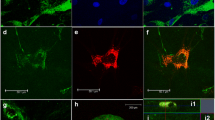
Tissue transglutaminase (tTG) is a multifunctional enzyme with a plethora of potential applications in regenerative medicine and tissue bioengineering. In this study, we examined the role of tTG as a regulator of chondrogenesis in human mesenchymal stem cells (MSC) using nanofibrous scaffolds coated with collagen type XI. Transient treatment of collagen type XI films and 3D scaffolds with tTG results in enhanced attachment of MSC and supports rounded cell morphology compared to the untreated matrices or those incubated in the continuous presence of tTG. Accordingly, enhanced cell aggregation and augmented chondrogenic differentiation have been observed on the collagen type XI-coated poly- (L-lactide) nanofibrous scaffolds treated with tTG prior to cell seeding. These changes implicate that MSC chondrogenesis is enhanced by the tTG-mediated modifications of the collagen matrix. For example, exogenous tTG increases resistance to collagenolysis in collagen type XI matrices by catalyzing intermolecular cross-linking, detected by a shift in the denaturation temperature. In addition, tTG auto-crosslinks to collagen type XI as detected by western blot and immunofluorescent analysis. This study identifies tTG as a novel regulator of MSC chondrogenesis further contributing to the expanding use of these cells in cartilage bioengineering.




Similar content being viewed by others
Abbreviations
- BSA:
-
Bovine serum albumin
- Ca2+ :
-
Calcium
- CO2 :
-
Carbon dioxide
- cont-tTG:
-
Collagen type XI films continuously treated with tTG
- pre-TG:
-
Collagen type XI films pre-treated with tTG
- DSC:
-
Differential scanning calorimetry
- DMEM:
-
Dulbecco’s modified eagle medium
- EDTA:
-
Ethylenediaminetetraacetic acid
- MSC:
-
Human mesenchymal stem cells
- HCl:
-
Hydrochloric acid
- PFA:
-
Paraformaldehyde
- PBS:
-
Phosphate buffered saline
- PLLA-coll XI:
-
PLLA scaffold coated with collagen type XI
- coll XI-pre tTG:
-
PLLA-coll XI pre-treated with tTG
- coll XI-cont tTG:
-
PLLA-coll XI in the continuous presence of tTG
- PLLA:
-
Poly (L-lactide)
- GAG:
-
Sulphated glycosaminoglycan
- 3D:
-
Three- dimensional
- tTG:
-
Tissue transglutaminase
- 2D:
-
Two-dimensional
References
Aeschlimann D, Thomazy V (2000) Protein crosslinking in assembly and remodelling of extracellular matrices: the role of transglutaminases. Connect Tissue Res 41(1):1–27. doi:10.3109/03008200009005638
Akagi A, Tajima S, Ishibashi A, Matsubara Y, Takehana M, Kobayashi S, Yamaguchi N (2002) Type XVI collagen is expressed in factor XIIIa+ monocyte-derived dermal dendrocytes and constitutes a potential substrate for factor XIIIa. J Invest Dermatol 118(2):267–274. doi:10.1046/j.0022-202x.2001.01666.x
Akimov SS, Krylov D, Fleischman LF, Belkin AM (2000) Tissue transglutaminase is an integrin-binding adhesion coreceptor for fibronectin. J Cell Biol. 21;148(4):825-38. doi:0021-9525/2000/02/825/14
Barsigian C, Stern AM, Martinez J (1991) Tissue (type II) transglutaminase covalently incorporates itself, fibrinogen, or fibronectin into high molecular weight complexes on the extracellular surface of isolated hepatocytes. Use of 2-[(2-oxopropyl)thio] imidazolium derivatives as cellular transglutaminase inactivators. J Biol Chem 266(33):22501–22509. Retrieved from http://www.jbc.org/
Birckbichler PJ, Orr GR, Carter HA, Patterson MK Jr (1977) Catalytic formation of epsilon-(gamma-glutamyl)lysine in guinea pig liver transglutaminase. Biochem Biophys Res Commun. 78(1):1–7. Retrieved from http://www.sciencedirect.com/science/journal/0006291X
Blaschke UK, Eikenberry EF, Hulmes DJ, Galla HJ, Bruckner P (2000) Collagen XI nucleates self-assembly and limits lateral growth of cartilage fibrils. J Biol Chem 275(14):10370–10378. doi:10.1074/jbc.275.14.10370
Bowness JM, Tarr AH, Wiebe RI (1989) Transglutaminase-catalysed cross-linking: a potential mechanism for the interaction of fibrinogen, low density lipoprotein and arterial type III procollagen. Thromb Res 54:357–367. doi:10.1016/0049-3848(89)90094-7
Cervellati C, Franzoni L, Squerzanti M, Bergamini CM, Spinozzi F, Mariani P, Lanzara V, Spisni A (2009) Unfolding studies of tissue transglutaminase. Amino Acids 36(4):633–641. doi:10.1007/s00726-008-0161-3
Chau DY, Collighan RJ, Verderio EA, Addy VL, Griffin M (2005) The cellular response to transglutaminase-cross-linked collagen. Biomaterials 26(33):6518–6529. doi:10.1016/j.biomaterials.2005.04.017
Christopher AM, Bailey AJ (1999) Thermal denaturation of collagen revisited. Proc Indian Acad Sci (Chem Sci) 111:71–80. doi:10.1007/BF02869897
Ciardelli G, Gentile P, Chiono V, Mattioli-Belmonte M, Vozzi G, Barbani N, Giusti P (2010) Enzymatically crosslinked porous composite matrices for bone tissue regeneration. J Biomed Mater Res A 92(1):137–151. doi:10.1002/jbm.a.32344
Dardik R, Inbal A (2006) Complex formation between tissue transglutaminase II (tTG) and vascular endothelial growth factor receptor 2 (VEGFR-2): proposed mechanism for modulation of endothelial cell response to VEGF. Exp Cell Res 312(16):2973–2982. doi:10.1016/j.yexcr.2006.05.019
Eyre DR, Weis MA, Wu JJ (2006) Articular cartilage collagen: an irreplaceable framework? Eur Cell Mater 12:57–63. Retrieved from http://www.ecmjournal.org/
Faverman L, Mikhaylova L, Malmquist J, Nurminskaya M (2008) Extracellular transglutaminase 2 activates beta-catenin signaling in calcifying vascular smooth muscle cells. FEBS Lett 582(10):1552–1557. doi:10.1016/j.febslet.2008.03.053
Fésüs L, Falus A, Erdei A, Laki K (1981) Human beta 2-microglobulin is a substrate of tissue transglutaminase: polymerization in solution and on the cell surface. J Cell Biol 89(3):706–710. doi:0021-9525/81/06/0706/05
Gao L, McBeath R, Chen CS (2010) Stem cell shape regulates a chondrogenic versus myogenic fate through Rac1 and N-cadherin. Stem Cells 28(3):564–572. doi:10.1002/stem.308
Garcia Y, Hemantkumar N, Collighan R, Griffin M, Rodriguez-Cabello JC, Pandit A (2009) In vitro characterization of a collagen scaffold enzymatically cross-linked with a tailored elastin-like polymer. Tissue Eng Part A. (4):887-99. doi:10.1089/ten.tea.2008.0104
Goldring MB, Tsuchimochi K, Ijiri K (2006) The control of chondrogenesis. J Cell Biochem 97(1):33–44. doi:10.1002/jcb.20652
Harris ED Jr, Farrell ME (1972) Resistance to collagenase: a characteristic of collagen fibrils cross-linked by formaldehyde. Biochim Biophys Acta. 1972 Aug 31;278(1):133–141. doi:10.1016/0005-2795(72)90114-6
Isobe T, Takahashi H, Ueki S, Takagi J, Saito Y (1999) Activity-independent cell adhesion to tissue-type transglutaminase is mediated by alpha4beta1 integrin. Eur J Cell Biol. 78(12):876-883. Retrieved from http://www.sciencedirect.com/science/journal/01719335
Jeleńska MM, Fesüs L, Kopeć M (1980) The comparative ability of plasma and tissue transglutaminases to use collagen as a substrate. Biochim Biophys Acta 616(2):167–178. doi:10.1016/0005-2744(80)90135-7
Jones RA, Nicholas B, Mian S, Davies PJ, Griffin M (1997) Reduced expression of tissue transglutaminase in a human endothelial cell line leads to changes in cell spreading, cell adhesion and reduced polymerisation of fibronectin. J Cell Sci. 110(Pt 19):2461–2472. Retrieved from http://jcs.biologists.org/
Jürgensen K, Aeschlimann D, Cavin V, Genge M, Hunziker EB (1997) A new biological glue for cartilage–cartilage interfaces: tissue transglutaminase. J Bone Joint Surg Am 79(2):185–193. Retrieved from http://www.jbjs.org/
Kleman JP, Aeschlimann D, Paulsson M, Van der Rest M (1995) Transglutaminase-catalyzed cross-linking of fibrils of collagen V/XI in A204 rhabdomyosarcoma cells. Biochemistry 34(42):13768–13775. doi:10.1021/bi00042a007
Lorand L, Graham RM (2003) Transglutaminases: crosslinking enzymes with pleiotropic functions. Nat Rev Mol Cell Biol 4(2):140–156. doi:10.1038/nrm1014
McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS (2004) Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell 6(4):483–495. doi:10.1016/S1534-5807(04)00075-9
Nakaoka H, Perez DM, Baek KJ, Das T, Husain A, Misono K, Im MJ, Graham RM (1994) Gh: a GTP-binding protein with transglutaminase activity and receptor signaling function. Science. 264(5165):1593–1596. doi:10.1126/science.7911253
Nurminsky D, Magee C, Faverman L, Nurminskaya M (2007) Regulation of chondrocyte differentiation by actin-severing protein adseverin. Dev Biol 302(2):427–437. doi:10.1016/j.ydbio.2006.09.052
Nurminsky D, Shanmugasundaram S, Deasey S, Michaud C, Allen S, Hendig D, Dastjerdi A, Francis-West P, Nurminskaya M (2010) Transglutaminase 2 regulates early chondrogenesis and glycosaminoglycan synthesis. Mech Dev doi:10.1016/j.mod.2010.11.007
Orban JM, Wilson LB, Kofroth JA, El-Kurdi MS, Maul TM, Vorp DA (2004) Crosslinking of collagen gels by transglutaminase. J Biomed Mater Res A 68(4):756–762. doi:10.1002/jbm.a.20110
Pelttari K, Wixmerten A, Martin I (2009) Do we really need cartilage tissue engineering? Swiss Med Wkly 139(41–42):602–609
Rimoin D, Francomano C, Giedion A (1998) International nomenclature and classification of the osteochondrodysplasias: international working group on constitutional diseases of bone. Am J Med Genet 79(5):376–382. doi:10.1002/(SICI)1096-8628(19981012)79:5<376:AID-AJMG9>3.0.CO;2-H
Shanmugasundaram S, Chaudhry H, Arinzeh TL (2011) Microscale versus nanoscale scaffold architecture for mesenchymal stem cell chondrogenesis. Tissue Eng Part A 17(5–6):831–840. doi:10.1089/ten.tea.2010.0409
Shrivastava A, Radziejewski C, Campbell E, Kovac L, McGlynn M, Ryan TE, Davis S, Goldfarb MP, Glass DJ, Lemke G, Yancopoulos GD (1997) An orphan receptor tyrosine kinase family whose members serve as non-integrin collagen receptors. Mol Cell 1:25–34. doi:10.1016/S1097-2765(00)80004-0
Song H, Chang W, Lim S, Seo HS, Shim CY, Park S, Yoo KJ, Kim BS, Min BH, Lee H, Jang Y, Chung N, Hwang KC (2007) Tissue transglutaminase is essential for integrin-mediated survival of bone marrow-derived mesenchymal stem cells. Stem Cells 25(6):1431–1438. doi:10.1634/stemcells.2006-0467
Spurlin TA, Bhadriraju K, Chung KH, Tona A, Plant AL (2009) The treatment of collagen fibrils by tissue transglutaminase to promote vascular smooth muscle cell contractile signaling. Biomaterials 30(29):5486–5496. doi:10.1016/j.biomaterials.2009.07.014
Telci D, Griffin M (2006) Tissue transglutaminase (TG2)-a wound response enzyme. Front Biosci. 11:867-82. doi: http://dx.doi.org/10.2741/1843
Verderio E, Coombes A, Jones RA, Li X, Heath D, Downes S, Griffin M (2001) Role of the cross-linking enzyme tissue transglutaminase in the biological recognition of synthetic biodegradable polymers. J Biomed Mater Res 54(2):294–304. doi:10.1002/1097-4636(200102)54:2<294:AID-JBM17>3.0.CO;2-Q
Woods A, Wang G, Beier F (2007) Regulation of chondrocyte differentiation by the actin cytoskeleton and adhesive interactions. J Cell Physiol 213(1):1–8. doi:10.1002/jcp.21110
Xu L, Begum S, Hearn JD, Hynes RO (2006) GPR56, an atypical G protein-coupled receptor, binds tissue transglutaminase, TG2, and inhibits melanoma tumor growth and metastasis. Proc Natl Acad Sci USA 103(24):9023–9028. doi:10.1073/pnas.0602681103
Xu J, Wang W, Ludeman M, Cheng K, Hayami T, Lotz JC, Kapila S (2008) Chondrogenic differentiation of human mesenchymal stem cells in 3D alginate gels. Tissue Eng Part A 14(5):667–680. doi:10.1089/tea.2007.0272
Acknowledgments
We would like to thank Dr. Kimberly Griswold, of Picatinny Arsenal, NJ for DSC measurements. Also, Dr. Michael Jaffe and Dr. George Collins of New Jersey Institute of Technology, NJ for helpful discussions on DSC data. This work was supported by NIH grants R56DK071920 and R03AR057126 and a grant from Maryland Stem Cell Research Fund to M. Nurminskaya.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
S. Shanmugasundaram, S. Logan-Mauney contributed equally.
Rights and permissions
About this article
Cite this article
Shanmugasundaram, S., Logan-Mauney, S., Burgos, K. et al. Tissue transglutaminase regulates chondrogenesis in mesenchymal stem cells on collagen type XI matrices. Amino Acids 42, 1045–1053 (2012). https://doi.org/10.1007/s00726-011-1019-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-011-1019-7