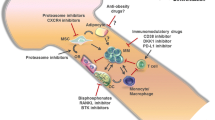
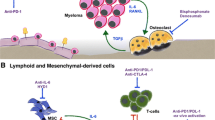
Abstract
Multiple myeloma (MM) is a plasma cell malignancy characterized by the frequent development of osteolytic lesions, osteopenia, pathological fractures, and/or severe bone pain. In the past few years several potential factors involved in this process have been identified and, with the increased knowledge of the signaling pathways involved in the regulation of normal osteoblast and osteoclast function, have provided us with a better understanding of the contributions of the marrow microenvironment to MM bone disease. These studies have identified several potential novel targets for treating MM bone disease in addition to the current standard treatment of bisphosphonates. In this article, we discuss several potential targets for treating MM bone disease as well as novel therapies that are in clinical trials for these patients.
Similar content being viewed by others
References
Kyle RA, Therneau TM, Rajkumar SV, Larson DR, Plevak MF, Melton LJ 3rd (2004) Incidence of multiple myeloma in Olmsted County, Minnesota: trend over 6 decades. Cancer (Phila) 101:2667–2674
Phekoo KJ, Schey SA, Richards MA, Bevan DH, Bell S, Gillett D, Moller H (2004) A population study to define the incidence and survival of multiple myeloma in a National Health Service Region in UK. Br J Haematol 127:299–304
American Cancer Society (2009) Cancer facts and figures. American Cancer Society, Atlanta, GA, pp 1–69
Callander NS, Roodman GD (2001) Myeloma bone disease. Semin Hematol 38:276–285
Melton LJ 3rd, Kyle RA, Achenbach SJ, Oberg AL, Rajkumar SV (2005) Fracture risk with multiple myeloma: a population-based study. J Bone Miner Res 20:487–493
Taube T, Beneton MN, McCloskey EV, Rogers S, Greaves M, Kanis JA (1992) Abnormal bone remodelling in patients with myelomatosis and normal biochemical indices of bone resorption. Eur J Haematol 49:192–198
Roodman GD (2004) Pathogenesis of myeloma bone disease. Blood Cells Mol Dis 32:290–292
Gordon S, Helfrich MH, Sati HI, Greaves M, Ralston SH, Culligan DJ, Soutar RL, Rogers MJ (2002) Pamidronate causes apoptosis of plasma cells in vivo in patients with multiple myeloma. Br J Haematol 119:475–483
Vinholes JJ, Purohit OP, Abbey ME, Eastell R, Coleman RE (1997) Relationships between biochemical and symptomatic response in a double-blind randomised trial of pamidronate for metastatic bone disease. Ann Oncol 8:1243–1250
Petcu EB, Schug SA, Smith H (2002) Clinical evaluation of onset of analgesia using intravenous pamidronate in metastatic bone pain. J Pain Symptom Manage 24:281–284
Markowitz GS, Appel GB, Fine PL, Fenves AZ, Loon NR, Jagannath S, Kuhn JA, Dratch AD, D’Agati VD (2001) Collapsing focal segmental glomerulosclerosis following treatment with high-dose pamidronate. J Am Soc Nephrol 12:1164–1172
Desikan R, Veksler Y, Raza S, Stokes B, Sabir T, Li ZJ, Jagannath S (2002) Nephrotic proteinuria associated with high-dose pamidronate in multiple myeloma. Br J Haematol 119:496–499
Gunn WG, Conley A, Deininger L, Olson SD, Prockop DJ, Gregory CA (2006) A crosstalk between myeloma cells and marrow stromal cells stimulates production of DKK1 and interleukin-6: a potential role in the development of lytic bone disease and tumor progression in multiple myeloma. Stem Cells 24:986–991
Choi SJ, Cruz JC, Craig F, Chung H, Devlin RD, Roodman GD, Alsina M (2000) Macrophage inflammatory protein 1-alpha is a potential osteoclast stimulatory factor in multiple myeloma. Blood 96:671–675
Giuliani N, Colla S, Rizzoli V (2004) New insight in the mechanism of osteoclast activation and formation in multiple myeloma: focus on the receptor activator of NF-kappaB ligand (RANKL). Exp Hematol 32:685–691
Lee JW, Chung HY, Ehrlich LA, Jelinek DF, Callander NS, Roodman GD, Choi SJ (2004) IL-3 expression by myeloma cells increases both osteoclast formation and growth of myeloma cells. Blood 103:2308–2315
Ehrlich LA, Roodman GD (2005) The role of immune cells and inflammatory cytokines in Paget’s disease and multiple myeloma. Immunol Rev 208:252–266
Sezer O, Heider U, Jakob C, Zavrski I, Eucker J, Possinger K, Sers C, Krenn V (2002) Immunocytochemistry reveals RANKL expression of myeloma cells. Blood 99:4646–4647
Giuliani N, Colla S, Sala R, Moroni M, Lazzaretti M, La Monica S, Bonomini S, Hojden M, Sammarelli G, Barille S, Bataille R, Rizzoli V (2002) Human myeloma cells stimulate the receptor activator of nuclear factor-kappa B ligand (RANKL) in T lymphocytes: a potential role in multiple myeloma bone disease. Blood 100:4615–4621
Pearse RN, Sordillo EM, Yaccoby S, Wong BR, Liau DF, Colman N, Michaeli J, Epstein J, Choi Y (2001) Multiple myeloma disrupts the TRANCE/osteoprotegerin cytokine axis to trigger bone destruction and promote tumor progression. Proc Natl Acad Sci USA 98:11581–11586
Menu E, Asosingh K, Van Riet I, Croucher P, Van Camp B, Vanderkerken K (2004) Myeloma cells (5TMM) and their interactions with the marrow microenvironment. Blood Cells Mol Dis 33:111–119
Epstein J, Yaccoby S (2005) The SCID-hu myeloma model. Methods Mol Med 113:183–190
Oba Y, Lee JW, Ehrlich LA, Chung HY, Jelinek DF, Callander NS, Horuk R, Choi SJ, Roodman GD (2005) MIP-1alpha utilizes both CCR1 and CCR5 to induce osteoclast formation and increase adhesion of myeloma cells to marrow stromal cells. Exp Hematol 33:272–278
Vallet S, Raje N, Ishitsuka K, Hideshima T, Podar K, Chhetri S, Pozzi S, Breitkreutz I, Kiziltepe T, Yasui H, Ocio EM, Shiraishi N, Jin J, Okawa Y, Ikeda H, Mukherjee S, Vaghela N, Cirstea D, Ladetto M, Boccadoro M, Anderson KC (2007) MLN3897, a novel CCR1 inhibitor, impairs osteoclastogenesis and inhibits the interaction of multiple myeloma cells and osteoclasts. Blood 110:3744–3752
Menu E, De Leenheer E, De Raeve H, Coulton L, Imanishi T, Miyashita K, Van Valckenborgh E, Van Riet I, Van Camp B, Horuk R, Croucher P, Vanderkerken K (2006) Role of CCR1 and CCR5 in homing and growth of multiple myeloma and in the development of osteolytic lesions: a study in the 5TMM model. Clin Exp Metastasis 23:291–300
Merico F, Bergui L, Gregoretti MG, Ghia P, Aimo G, Lindley IJ, Caligaris-Cappio F (1993) Cytokines involved in the progression of multiple myeloma. Clin Exp Immunol 92:27–31
Barton BE, Mayer R (1989) IL-3 induces differentiation of bone marrow precursor cells to osteoclast-like cells. J Immunol 143:3211–3216
Solary E, Guiguet M, Zeller V, Casasnovas RO, Caillot D, Chavanet P, Guy H, Mack G (1992) Radioimmunoassay for the measurement of serum IL-6 and its correlation with tumour cell mass parameters in multiple myeloma. Am J Hematol 39:163–171
Sati HI, Apperley JF, Greaves M, Lawry J, Gooding R, Russell RG, Croucher PI (1998) Interleukin-6 is expressed by plasma cells from patients with multiple myeloma and monoclonal gammopathy of undetermined significance. Br J Haematol 101:287–295
Abildgaard N, Glerup H, Rungby J, Bendix-Hansen K, Kassem M, Brixen K, Heickendorff L, Nielsen JL, Eriksen EF (2000) Biochemical markers of bone metabolism reflect osteoclastic and osteoblastic activity in multiple myeloma. Eur J Haematol 64:121–129
Karadag A, Oyajobi BO, Apperley JF, Russell RG, Croucher PI (2000) Human myeloma cells promote the production of interleukin 6 by primary human osteoblasts. Br J Haematol 108:383–390
Abe M, Hiura K, Wilde J, Shioyasono A, Moriyama K, Hashimoto T, Kido S, Oshima T, Shibata H, Ozaki S, Inoue D, Matsumoto T (2004) Osteoclasts enhance myeloma cell growth and survival via cell–cell contact: a vicious cycle between bone destruction and myeloma expansion. Blood 104:2484–2491
Anderson KC, Jones RM, Morimoto C, Leavitt P, Barut BA (1989) Response patterns of purified myeloma cells to hematopoietic growth factors. Blood 73:1915–1924
Ehrlich LA, Chung HY, Ghobrial I, Choi SJ, Morandi F, Colla S, Rizzoli V, Roodman GD, Giuliani N (2005) IL-3 is a potential inhibitor of osteoblast differentiation in multiple myeloma. Blood 106:1407–1414
Giuliani N, Colla S, Morandi F, Lazzaretti M, Sala R, Bonomini S, Grano M, Colucci S, Svaldi M, Rizzoli V (2005) Myeloma cells block RUNX2/CBFA1 activity in human bone marrow osteoblast progenitors and inhibit osteoblast formation and differentiation. Blood 106:2472–2483
Oshima T, Abe M, Asano J, Hara T, Kitazoe K, Sekimoto E, Tanaka Y, Shibata H, Hashimoto T, Ozaki S, Kido S, Inoue D, Matsumoto T (2005) Myeloma cells suppress bone formation by secreting a soluble Wnt inhibitor, sFRP-2. Blood 106:3160–3165
Tian E, Zhan F, Walker R, Rasmussen E, Ma Y, Barlogie B, Shaughnessy JD Jr (2003) The role of the Wnt-signaling antagonist DKK1 in the development of osteolytic lesions in multiple myeloma. N Engl J Med 349:2483–2494
Canalis E, Deregowski V, Pereira RC, Gazzerro E (2005) Signals that determine the fate of osteoblastic cells. J Endocrinol Invest 28:3–7
Westendorf JJ, Kahler RA, Schroeder TM (2004) Wnt signaling in osteoblasts and bone diseases. Gene (Amst) 341:19–39
Bain G, Muller T, Wang X, Papkoff J (2003) Activated beta-catenin induces osteoblast differentiation of C3H10T1/2 cells and participates in BMP2 mediated signal transduction. Biochem Biophys Res Commun 301:84–91
Qiang YW, Chen Y, Stephens O, Brown N, Chen B, Epstein J, Barlogie B, Shaughnessy JD Jr (2008) Myeloma-derived Dickkopf-1 disrupts Wnt-regulated osteoprotegerin and RANKL production by osteoblasts: a potential mechanism underlying osteolytic bone lesions in multiple myeloma. Blood 112:196–207
Politou MC, Heath DJ, Rahemtulla A, Szydlo R, Anagnostopoulos A, Dimopoulos MA, Croucher PI, Terpos E (2006) Serum concentrations of Dickkopf-1 protein are increased in patients with multiple myeloma and reduced after autologous stem cell transplantation. Int J Cancer 119:1728–1731
Kaiser M, Mieth M, Liebisch P, Oberlander R, Rademacher J, Jakob C, Kleeberg L, Fleissner C, Braendle E, Peters M, Stover D, Sezer O, Heider U (2008) Serum concentrations of DKK-1 correlate with the extent of bone disease in patients with multiple myeloma. Eur J Haematol 80:490–494
Zangari M, Esseltine D, Cavallo F, Neuwirth R, Elice F, Burns MJ, Yaccoby S, Richardson P, Sonneveld P, Tricot G (2007) Predictive value of alkaline phosphatase for response and time to progression in bortezomib-treated multiple myeloma patients. Am J Hematol 82:831–833
Oshima T, Abe M, Asano J, Hara T, Kitazoe K, Sekimoto E, Tanaka Y, Shibata H, Hashimoto T, Ozaki S, Kido S, Inoue D, Matsumoto T (2004) Myeloma cells suppress osteoblast differentiation by secreting a soluble wnt inhibitor, sFRP-2. American Society of Hematology, San Diego, CA (abstract 2356)
De Vos J, Couderc G, Tarte K, Jourdan M, Requirand G, Delteil MC, Rossi JF, Mechti N, Klein B (2001) Identifying intercellular signaling genes expressed in malignant plasma cells by using complementary DNA arrays. Blood 98:771–780
Giuliani N, Morandi F, Tagliaferri S, Lazzaretti M, Donofrio G, Bonomini S, Sala R, Mangoni M, Rizzoli V (2007) Production of Wnt inhibitors by myeloma cells: potential effects on canonical Wnt pathway in the bone microenvironment. Cancer Res 67:7665–7674
Davies FE, Dring AM, Li C, Rawstron AC, Shammas MA, O’Connor SM, Fenton JA, Hideshima T, Chauhan D, Tai IT, Robinson E, Auclair D, Rees K, Gonzalez D, Ashcroft AJ, Dasgupta R, Mitsiades C, Mitsiades N, Chen LB, Wong WH, Munshi NC, Morgan GJ, Anderson KC (2003) Insights into the multistep transformation of MGUS to myeloma using microarray expression analysis. Blood 102:4504–4511
Weitzmann MN, Roggia C, Toraldo G, Weitzmann L, Pacifici R (2002) Increased production of IL-7 uncouples bone formation from bone resorption during estrogen deficiency. J Clin Invest 110:1643–1650
Giuliani N, Rizzoli V (2007) Myeloma cells and bone marrow osteoblast interactions: role in the development of osteolytic lesions in multiple myeloma. Leuk Lymphoma 48:2323–2329
Body JJ, Facon T, Coleman RE, Lipton A, Geurs F, Fan M, Holloway D, Peterson MC, Bekker PJ (2006) A study of the biological receptor activator of nuclear factor-kappaB ligand inhibitor, denosumab, in patients with multiple myeloma or bone metastases from breast cancer. Clin Cancer Res 12:1221–1228
Vij R, Horvath N, Spencer A, Taylor K, Saroj V, Smith J, Qian Y, Jun S (2007) An open label phase 2 trial of Denosumab in the treatment of relapsed or plateau-phase myeloma. Blood 118:1054A
Henry D, von Moos R, Vadhan-Raj S, Hungria V, Spencer A, Hirsh V, Wang J, Jun S, Yeh H, Dansey R (2009) A double-blind, randomized study of denosumab versus zoledronic acid for the treatment of bone metastases in patients with advanced cancer (excluding breast and prostate cancer) or multiple myeloma. Eur J Cancer Suppl 7:12 (abstract)
von Metzler I, Krebbel H, Hecht M, Manz RA, Fleissner C, Mieth M, Kaiser M, Jakob C, Sterz J, Kleeberg L, Heider U, Sezer O (2007) Bortezomib inhibits human osteoclastogenesis. Leukemia 21:2025–2034
Terpos E, Sezer O, Croucher P, Dimopoulos MA (2007) Myeloma bone disease and proteasome inhibition therapies. Blood 110:1098–1104
Ozaki S, Tanaka O, Fujii S, Shigekiyo Y, Miki H, Choraku M, Kagawa K, Asano J, Takeuchi K, Kitazoe K, Hashimoto T, Abe M, Matsumoto T (2007) Therapy with bortezomib plus dexamethasone induces osteoblast activation in responsive patients with multiple myeloma. Int J Hematol 86:180–185
Giuliani N, Morandi F, Tagliaferri S, Lazzaretti M, Bonomini S, Crugnola M, Mancini C, Martella E, Ferrari L, Tabilio A, Rizzoli V (2007) The proteasome inhibitor bortezomib affects osteoblast differentiation in vitro and in vivo in multiple myeloma patients. Blood 110:334–338
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Roodman, G.D. Targeting the bone microenvironment in multiple myeloma. J Bone Miner Metab 28, 244–250 (2010). https://doi.org/10.1007/s00774-009-0154-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-009-0154-7