Abstract
To clarify the genotype–phenotype correlation of 5p- syndrome, FISH analyses were performed for six patients by using a series of probes spanning 5p13.1–p15.33. Genotypically, break points of deletion were quite different. Three of the six patients were diagnosed as interstitial deletion on chromosome 5p by G-banding method and FISH analysis; however, all of them proved to be entire distal deletions of 5p caused by unbalanced chromosomal translocations. Furthermore, one 5p- syndrome patient was diagnosed only by the FISH analysis using a single probe but not by ordinary chromosomal analyses. Therefore, when ordinary chromosomal analysis cannot detect any deletion in a patient who is phenotypically suspected of 5p- syndrome, multiple FISH analysis or parental chromosomal analysis would be needed for correct diagnosis. Interestingly, one patient with terminal deletion between 5p15.31-pter lacks mental retardation and cat-like crying, indicating that this region might not be responsible for those cardinal features of 5p- syndrome. Further studies on genotype–phenotype correlation will help us better understand 5p- syndrome and also determine functional mapping of the 5p region.
Similar content being viewed by others
Introduction
5p- syndrome, also called “Cat cry syndrome” or “Cri du chat syndrome,” varies clinically and genetically (Mainardi et al. 2001). Recent molecular cytogenetic analyses have defined 5p15.3 and 5p15.2 as responsible for “cat-like cry” and “dysmorphism, microcephaly, and mental retardation,” respectively (Gersh et al. 1995). On the other hand, Mainardi et al. (2001) reported that the region for cat-like cry was located between D5S13 and D5S731. We report here the detailed chromosomal analysis of six 5p- syndrome patients. One of them lacked such characteristic manifestations as mental retardation and cat-like cry despite the terminal small deletion of chromosome 5p. The other had typical clinical features, but her karyotype was reported to be normal by the ordinary G-banding method. Our experience emphasizes the importance of detailed chromosomal analysis in investigation of phenotype–genotype correlation.
Subjects and methods
Patients
Clinical features of six patients are listed in Table 1. All patients had growth retardation, hypotonia, and epicanthal fold. Patients 1, 2, and 4 had clinical features typical of 5p- syndrome. Patients 3 and 5 lacked severe mental retardation, although patient 3 was developmentally delayed. Her neck control and walking without support were achieved at 7 and 18 months of age, respectively. However, her facial expression and crying voice were not characteristic of 5p- syndrome. Chromosomal examination demonstrated that her karyotype was 46,XX,del(5)(p15.3). She is now 6 years old, and her present IQ is 90. She could also catch up with motor development. Patient 5 had intrauterine growth retardation (IUGR), hypotonia, and severe bilateral talipes valgus. Her karyotype was 46,XX,del(5)(p14p15.2). Her total DQ was 45 at 3 years when she could speak some single words. Patient 6 was clinically typical of 5p- syndrome, presenting with IUGR, hypotonia, cat-like crying, severe bilateral talipes valgus, and peculiar facies. Although her karyotype was reported to be normal by the ordinary G-banding method (Fig. 1a), fluorescence in situ hybridization (FISH) analysis using a probe D5S23 targeting 5p15.2 demonstrated chromosomal microdeletion of 5p15.2 (Fig. 1b). Parental chromosomal investigations were performed in patients 1–4. All showed normal karyotype. Parents of patients 5 and 6 deferred their cytogenetic examinations.
FISH analysis
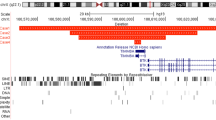
To elucidate the fine structural changes in 5p, FISH analysis was performed according to the standard protocol (Shimokawa et al. 2004). Twenty-eight BAC clones mapped on 5p13.1–p15.33 were obtained according to Human Genome Browser July 2003 version (Fig. 2). Terminal analyses of 5p were not performed.
Results and discussion
FISH analysis
Break points varied among the six patients (Fig. 2). In patients 3, 5, and 6, who had been suspected of the interstitial deletion of 5p by ordinary chromosomal analysis, deletion of the entire distal short arm of chromosome 5 was demonstrated. These results indicate that their chromosomes 5 are derivatives resulting from unbalanced translocations. The derivative chromosome 5 of patient 6 was further examined by the whole chromosome painting method, and the size of a large deletion in chromosome 5 was estimated (Fig. 1c). Thus, FISH analysis would be essential for correct diagnosis of index patients, and prenatal diagnosis in the future, when patients are suspected to have interstitial deletion in chromosome 5p or are reported to have normal karyotype by ordinary G-banding method.
Genotype–phenotype correlation
The summary of genotypes and phenotypes of six patients is shown with chromosomal map (Fig. 2). Grossly, the severity and spectrum of clinical features (phenotypes) apparently depend on the size and location of the deletion (genotypes). Because patient 3 lacked cat-like cry and mental retardation, the region responsible for cat-like cry may be located on the proximal part to RP11-91M19 located on 5p15.31. Furthermore, the region distal from RP11-91M19 does not appear to be critical for mental retardation. The delta-catenin gene was reported to be important in the developmental abnormalities and expressed early in neuronal development (Medina et al. 2000). Since the corresponding region (Rp11-79L17) was conserved in patient 3, this gene might be essential for mental conditions. Zhang et al. (2003) reported that the deletion of the hTERT gene located on 5p15.33 in 5p- syndrome might impair normal fetal development. However, we have shown that patients 1, 4, and 5, who lacked IUGR, as well as those with IUGR, lost hTERT. These results are against the role of hTERT gene in normal fetal development.
Two patients, 5 and 6, who had unbalanced translocation, were complicated with severe talipes valgus. Further studies are needed to clarify whether this complication is related with 5p- syndrome or not. Thus, as long as precise genetic diagnosis is made, genotypes and phenotypes appear to correlate with each other. Although multiple FISH analysis is not feasible, it should be considered for better management and understanding of 5p- syndrome.
References
Church DM, Yang J, Bocian M, Shiang R, Wasmuth JJ (1997) A high-resolution physical and transcript map of cri du chat region of human chromosome 5p. Genome Res 7:787–801
Gersh M, Goodart SA, Pasztor LM, Harris DJ, Weiss L, Overhauser J (1995) Evidence for a distinct region causing a cat-like cry in patients with 5p deletions. Am J Hum Genet 56:1404–1410
Mainardi PC, Perfumo C, Cali A, Coucourde G, Pastore G, Cavani S, Zara F, Overhauser J, Pierluigi M, Bricarelli FD (2001) Clinical and molecular characterisation of 80 patients with 5p deletion: genotype–phenotype correlation. J Med Genet 38:151–158
Medina M, Marinescu RC, Overhauser J, Kosik KS (2000) Hemizygosity of delta- catenin (CTNND2) is associated with severe mental retardation in cri-du-chat syndrome. Genomics 63:157–164
Shimokawa O, Kurosawa K, Ida T, .Harada N, Kondoh T, Miyake N, Yoshiura K, Kishino T, Ohta T, Niikawa N, Matsumoto N (2004) Molecular characterization of inv dup del(8p): analysis of five cases. Am J Med Genet 128A:133–137
Zhang A, Zheng C, Hou M, Lindvall C, Ki KJ, Erlandsson F, Bjorkholm M, Gruber A, Blennow E, Xu D (2003) Deletion of the telomerase reverse transcriptase gene and haploinsufficiency of telomere maintenance in Cri du chat syndrome. Am J Hum Genet 72:940–948
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kondoh, T., Shimokawa, O., Harada, N. et al. Genotype-phenotype correlation of 5p- syndrome: pitfall of diagnosis. J Hum Genet 50, 26–29 (2005). https://doi.org/10.1007/s10038-004-0213-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10038-004-0213-9
Keywords
This article is cited by
-
Prenatal diagnosis of cri-du-chat syndrome by SNP array: report of twelve cases and review of the literature
Molecular Cytogenetics (2019)
-
A cryptic balanced translocation (5;17), a puzzle revealed through a critical evaluation of the pedigree and a FISH focused on candidate loci suggested by the phenotype
Molecular Cytogenetics (2015)
-
Use of Multiplex Ligation-Dependent Probe Amplification (MLPA) in screening of subtelomeric regions in children with idiopathic mental retardation
The Indian Journal of Pediatrics (2009)
-
Cri du Chat syndrome
Orphanet Journal of Rare Diseases (2006)