Abstract
The objective of this study is to assess patients’ satisfaction with migraine treatment with frovatriptan (F) or zolmitriptan (Z), by preference questionnaire. 133 subjects with a history of migraine with or without aura (IHS criteria) were randomized to F 2.5 mg or Z 2.5 mg. The study had a multicenter, randomized, double-blind, cross-over design, with each of the two treatment periods lasting no more than 3 months. At the end of the study, patients were asked to assign preference to one of the treatments (primary endpoint). The number of pain-free (PF) and pain-relief (PR) episodes at 2 h, and number of recurrent and sustained pain-free (SPF) episodes within 48 h were the secondary study endpoints. Seventy-seven percent of patients expressed a preference. Average score of preference was 2.9 ± 1.3 (F) versus 3.0 ± 1.3 (Z; p = NS). Rate of PF episodes at 2 h was 26% with F and 31% with Z (p = NS). PR episodes at 2 h were 57% for F and 58% for Z (p = NS). Rate of recurrence was 21 (F) and 24% (Z; p = NS). Time to recurrence within 48 h was better for F especially between 4 and 16 h (p < 0.05). SPF episodes were 18 (F) versus 22% (Z; p = NS). Drug-related adverse events were significantly (p < 0.05) less under F (3 vs. 10). In conclusion, our study suggests that F has a similar efficacy of Z, with some advantage as regards tolerability and recurrence.
Similar content being viewed by others
Introduction
Triptans are generally considered as the most effective acute treatment for migraine [1]. The therapeutic success of sumatriptan, the parent drug of this class, in the treatment of this neurological condition [2] has prompted the development of other triptan compounds, trying to optimize efficacy and safety in migraine management.
Frovatriptan (F) is one of the newest triptans, developed in order to provide a clinical potential for a long duration of action and a low likelihood of side effects and drug interactions [3]. However, with the exception of one study versus sumatriptan [4], there are presently no head-to-head randomized trials comparing efficacy and safety of F with that of other triptans. For this reason, a study was setup to compare efficacy and safety of F versus zolmitriptan (Z), a triptan widely employed as first-line therapy for migraine [5].
The study has been designed to assess efficacy by analyzing traditional migraine treatment endpoints and also by considering patient’s preference to treatment [6].
Methods
Study population and design
Male or female subjects, aged 18–65 years, with a current history of migraine with or without aura, according to IHS criteria, and with at least one migraine attack per month for 6 months prior to entering the study, were eligible for participation in the study [7].
Patients with uncontrolled hypertension, cardiac, vascular, liver and renal impairment, or any other severe or disabling medical condition could not be enrolled. Individuals with history of alcohol or analgesic or psychotropic drug abuse, known hypersensitivity to study drugs, previous inadequate response to at least two triptans, currently using ergotamine (and its derivatives) or MAO-inhibitors, or suffering from headaches that have been lasting for >6 days, were excluded as well. Pregnant women, breast-feeding mothers, and women with childbearing potential having a positive or missing pregnancy test were not eligible.
Written informed consent was obtained from all patients prior to their inclusion in the study. The study was approved by the Independent Institutional Review Boards of the study centers.
The study had a multicenter, randomized, double-blind, cross-over design, and included 14 Italian centers (Appendix 1). Each patient received F 2.5 mg or Z 2.5 mg in a randomized sequence. After treating three episodes of migraine in not >3 months with the first treatment, the patient had to switch to the other treatment. After treating three episodes of migraine in not >3 months with the second treatment, each patient was asked to assign preference to one of the treatments according to a questionnaire with a preference score graded from 0 to 5 on a 10-cm scale.
Subjects were instructed to treat at least three migraine episodes occurring in not >3 months and to come for the second visit and to take one dose of study medication as early as possible after the onset of migraine attack. If insufficient relief had been obtained after 2 h, patients were allowed to take a second dose of study medication, with a maximum daily intake of two doses. In case of insufficient relief 1 h after the intake of the second dose of the study medication, patients were allowed to take a rescue medication (excluding other triptans, ergotamine or its derivatives).
During the study use of concomitant medications, occurrence of adverse events (from diary), blood pressure, and heart rate were regularly checked, and a physical and neurological examination performed. A headache diary was dispensed with study medication.
Data analysis
The primary study endpoint was the between-treatment comparison of the direction and average strength of preference at the end of the study, measured on a scale from 0 to 5. The hypothesis was that a superiority of one treatment against the other had to occur in the presence of a difference of +1.0 with a standard deviation of 2.375. Considering a two-tailed test with a 0.05 significance level and an 80.7% power, the estimated number of patients to be randomized was 120 (including a 25% of drop-outs), 60 for each treatment group.
The intention-to-treat population (ITT, all patients treating at least one attack in each treatment period and completing the preference questionnaire) was the study primary analysis population, while the per-protocol population was the confirmatory analysis.
Secondary study endpoints were quantified according to IHS Guidelines [7] (1) pain-free (PF) episodes at 2 h (absence of migraine 2 h after intake of one dose of study drug and without any rescue medication), (2) recurrence (migraine occurring within 48 h after a period without migraine), (3) sustained pain-free (SPF) episodes within 48 h (migraine attack which is PF at 2 h, does not recur and does not require the use of rescue medication or a second study drug dose within 48 h), and (4) pain-relief (PR) episodes at 2 h (defined as a decrease in migraine intensity from severe or moderate to mild or none). Consistency of recurrence, defined as patients having at least two or three recurrences over three attacks, was also assessed.
Safety analysis was applied to all randomized patients, by calculating the incidence of adverse events and changes in vital signs during the study.
Preference scores were compared between treatment groups by analysis of variance, while logistic regression analysis was used for testing the difference in the proportion of patients with preference for one of the two drugs. Secondary endpoints were compared between groups by generalized estimating equation analysis. The level of statistical significance was kept at 0.05 throughout the whole study.
Results
Baseline demographic and clinical data
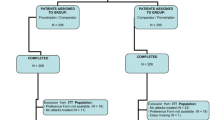
A total of 133 patients were screened and randomized to active treatment, of which 105 completed and 28 discontinued the study.
The ITT population consisted of 107 patients (Table 1): 68 patients were valid for per-protocol analysis and 121 for safety analysis.
Patient preference
Seventy-seven percent of patients expressed a preference for a triptan. Average preference score was 2.9 ± 1.3 with F and 3.0 ± 1.3 with Z (p = NS). The average score was 3.2 ± 1.1 for F and 3.2 ± 1.2 for Z when values between 0 and 1 were considered as no preference and 5 as strong preference. Most common reasons for preferring either triptan were rapid activity (83% F vs. 72% Z), reduction of headache severity (53 vs. 42%), and no side effects (40 vs. 40%). Additional preference results will be published in detail elsewhere.
Secondary end-points
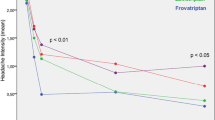
Thirty-four percent of patients preferred F while 43% preferred Z (p = NS). Rate of PF episodes at 2 h (26 vs. 31%) was similar (p = NS) for F and Z, as well as the rate of recurrent episodes (21 vs. 24%), SPF episodes (18 vs. 22%), and PR episodes at 2 h (57 vs. 58%) (Table 2). The risk of recurrence over the 48 h was lower with F, especially between 4 and 16 h (p < 0.05) (Fig. 1). Recurrence of mild intensity attacks was lower for F (17%) than for Z (37%) (p < 0.05) as well as consistency of recurrence over all the attacks (13% F vs. 20% Z; p < 0.05).
Safety
Thirty-one adverse events were recorded (12 under F and 19 under Z). Side effects attributed to study treatment were 13 and occurred significantly (p < 0.05) more often in Z- (n = 10) than in F-treated patients (n = 3); six events in Z-treated patients versus none in F-treated patients has a sever intensity (Table 3). No patient reported angina-like symptoms (tachycardia, thoracic constriction, or pain) in the F group versus four in the Z group (Table 3).
Discussion
This is the first direct head-to-head comparative study of F with another triptan, strictly applying IHS criteria for definition of study endpoints. When using these traditional endpoints, Z and F resulted in a similar efficacy. This difference did not seem to influence patient preference for one drug or the other. Interestingly, the frequency of 48-h SPF episodes was similar between the two triptans, though a significantly lower rate of recurrence was observed under F in the first 4–16 h from drug intake.
According to results of patient’s preference analysis, F was chosen mainly because of the rapid speed of onset of action (83% of patients) and the reduction in pain severity (53% of patients): 40% of patients appreciated its good tolerability.
Previous direct comparisons between F and Z are not available. However, our results are in line with those of previous studies based on Z [8–11]. As far as F is regarded, previous randomized, double-blind, placebo-controlled studies, showed a lower PR rate at 2 h with F as respect to our study (38–40 vs. 57%) [3]. The additional finding of our study is that proportion of PF episodes at 2 h was much higher than that observed in previous placebo-controlled studies (26 vs. 9–14%) [10].
F showed a similar efficacy and patient’s preference, while appeared to be safer than Z, with a significantly lower rate of drug-related adverse events.
In conclusion, our multicenter, randomized, double-blind trial, supports the validity of the patient preference approach for the evaluation of migraine treatment, and suggests that, in spite of a similar efficacy, on the long-term, F may have some advantage on Z in terms of safety.
References
Rapoport AM (2006) Acute treatment of headache. J Headache Pain 7:355–359
Cady R, Schreiber C (2006) Sumatriptan: update and review. Expert Opin Pharmacother 7:1503–1514
Balbisi EA (2004) Frovatriptan succinate, a 5-HT1B/1D receptor agonist for migraine. Int J Clin Pract 58:695–705
Géraud G, Spierings EL, Keywood C (2002) Tolerability and safety of frovatriptan with short- and long-term use for treatment of migraine and in comparison with sumatriptan. Headache 42(Suppl 2):S93–S99
Dowson AJ, Charlesworth B (2002) Review of zolmitriptan and its clinical applications in migraine. Expert Opin Pharmacother 3:993–1005
Dahlöf C (2001) Assessing patient preference in migraine treatment. Cephalalgia 21:791–795
International Headache Society Clinical Trials Subcommittee (2000) Guidelines for controlled trials of drugs in migraine: second edition. Cephalalgia 20:765–786
Ferrari MD, Goadsby PJ, Roon KI, Lipton RB (2002) Triptans (serotonin, 5-HT1B/1D agonists) in migraine: detailed results and methods of a meta-analysis of 53 trials. Cephalalgia 22:633–658
Adelman JU, Belsey J (2003) Meta-analysis of oral triptan therapy for migraine: number needed to treat and relative cost to achieve relief within 2 hours. J Manag Care Pharm 9:45–52
Géraud G, Keywood C, Senard JM (2003) Migraine headache recurrence: relationship to clinical, pharmacological, and pharmacokinetic properties of triptans. Headache 43:376–388
Chen LC, Ashcroft DM (2008) Meta-analysis of the efficacy and safety of zolmitriptan in the acute treatment of migraine. Headache 48:236–247
Acknowledgments
The present study was supported by Istituto Lusofarmaco d’Italia.
Conflict of interest
All authors have occasionally served as scientific consultants for manufacturers of frovatriptan or zolmitriptan.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Appendix 1: list of study sites
Appendix 1: list of study sites
Coordinator: G. Bussone (Milano).
Investigators: M. Gionco (Torino), A. Aguggia (Novi Ligure), B. Colombo (Milano), M. Turla (Esine), F. Perini (Vicenza), A. Ganga (Sassari), E. Agostoni (Lecco), C. Narbone (Messina), A. Moschiano (Merate), M. Vacca (Cagliari), M. Bartolini (Ancona), A. Ambrosini (Pozzilli), R. De Simone (Napoli), V. Petretta, F. D’Onofrio (Avellino), G. Reggiardo (Biostatistical Unit, Mediservice, Milano), F. Sacchi (Clinical Unit, Mediservice, Milano).
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Tullo, V., Allais, G., Ferrari, M.D. et al. Frovatriptan versus zolmitriptan for the acute treatment of migraine: a double-blind, randomized, multicenter, Italian study. Neurol Sci 31 (Suppl 1), 51–54 (2010). https://doi.org/10.1007/s10072-010-0273-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-010-0273-x