Abstract
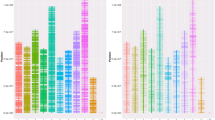
The eastern oyster, Crassostrea virginica, and the Pacific oyster, C. gigas, are species of global economic significance as well as important components of estuarine ecosystems and models for genetic and environmental studies. To enhance the molecular tools available for oyster research, an international group of collaborators has constructed a 27,496-feature cDNA microarray containing 4460 sequences derived from C. virginica, 2320 from C. gigas, and 16 non-oyster DNAs serving as positive and negative controls. The performance of the array was assessed by gene expression profiling using gill and digestive gland RNA derived from both C. gigas and C. virginica, and digestive gland RNA from C. ariakensis. The utility of the microarray for detection of homologous genes by cross-hybridization between species was also assessed and the correlation between hybridization intensity and sequence homology for selected genes determined. The oyster cDNA microarray is publicly available to the research community on a cost-recovery basis.









Similar content being viewed by others
References
Adjaye J, Herwig A, Herrmann D, Wruck W, BenKahla A, Brink TC, Nowak M, Carnwath JW, Hultschig C, Niemann H, Lehrach H (2004) Cross-species hybridisation of human and bovine orthologous genes on high density cDNA microarrays. BMC Genomics 5, 83
Ashburner M, Ball AC, Blake JA, Botstein D, Butler H, Cherry M, Davis AP, Dolinski K, Dwight SS, Eppig JT, Harris MA, Hill DP, Issel-Tarver L, Kasarskis A, Lewis S, Matese JC, Richardson JE, Ringwald M, Rubin GM, Sherlock G (2000) Gene ontology: tool for the unification of biology. Nat Genet 25, 25–29
Benjamini Y, Holchberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Statist Soc B 57, 289–300
Boutet I, Tanguy A, Moraga D (2004) Response of the Pacific oyster Crassostrea gigas to hydrocarbon contamination under experimental conditions. Gene 329, 147–157
Chen YA, McKillen DJ, Wu S, Jenny MJ, Chapman RW, Gross PS, Warr GW, Almeida JS (2004) Optimal cDNA microarray design using expressed sequence tags for organisms with limited genomic information. BMC Bioinformatics 5, 191
Chen Y, Chou C-C, Lu X, Slate E, Peck K, Xu W, Voit E, Almeida J (2006) A multivariate prediction model for microarray cross-hybridization. BMC Bioinformatics 7, 101
Cheney DP, MacDonald BF, Elston RA (2000) Summer mortality of Pacific oysters, Crassostrea gigas (Thunberg): initial finding on multiple environmental stressors in Puget Sound, Washington, 1998. J Shellfish Res 19, 353–359
Cunningham C, Hikima J, Jenny MJ, Chapman RW, Fang G-C, Saski C, Lundqvist M, Wing RA, Cupit PM, Gross PS, Warr RW, Tomkins JP (2006) New resources for marine genomics: bacterial artificial chromosome libraries for the eastern and Pacific oysters (Crassostrea virginica and C gigas). Mar Biotechnol 8, 521–533
David E, Tanguy A, Pichavant K, Moraga D (2005) Response of the Pacific oyster Crassostrea gigas to hypoxia exposure under experimental conditions. FEBS J 272, 5635–5652
de Lorgeril J, Saulnier D, Janech MG, Guegen Y, Bachere E (2005) Identification of genes that are differentially expressed in hemocytes of the Pacific blue shrimp (Litopenaeus stylirostris) surviving an infection with Vibrio penaeicida. Physiol Genomics 21, 174–183
Goulletquer P, Soletchnik P, Le Moine O, Razet D, Geairon P, Faury N, Taillade S (1998) Summer mortality of the Pacific cupped oysters Crassostrea gigas in the Bay of Marennes-Oleron (France). Cons Int Explor Mer CIEM 14, 20
Gracey AY, Troll JV, Somero GN (2001) Hypoxia-induced gene expression profiling in the euryoxic fish Gillichthys mirabilis. Proc Natl Acad Sci USA 98, 1993–1998
Gueguen Y, Cadoret JP, Flament D, Barreau-Roumiguiere C, Girardot AL, Garnier J, Hoareau A, Bachere E, Escoubas JM (2003) Immune gene discovery by expressed sequence tags generated from hemocytes of the bacteria-challenged oyster, Crassostrea gigas. Gene 303, 139–145
Hedgecock D, Gaffney PM, Goulletquer P, Guo X, Reece K, Warr GW (2005) The case for sequencing the Pacific oyster genome. J Shellfish Res 24, 429–441
Huber W, von Heydebreck A, Sultmann H, Poustka A, Vingron M (2002) Variance stabilization applied to microarray data calibration and to the quantification of differential expression. Bioinformatics 18(Suppl 1):S96–S104
Huvet A, Herpin A, Degremont L, Labreuche Y, Samain J-F, Cunningham C (2004) The identification of genes from the oyster Crassostrea gigas that are differentially expressed in progeny exhibiting opposed susceptibility to summer mortality. Gene 343, 211–220
Jenny MJ, Ringwood AH, Lacy ER, Lewitus AJ, Kempton JW, Gross PS, Warr GW, Chapman, RW (2002) Potential indicators of stress response identified by expressed sequence tag analysis of hemocytes and embryos from the American oyster, Crassostrea virginica. Mar Biotechnol 4, 81–93
Kleinbaum D, Kupper L, Muller K, Nizam A (1998) Applied Regression Analysis and Other Multivariable Methods, 3rd ed (Pacific Grove, CA: Duxbury)
Koganezawa A (1974) Present status of studies on the mass mortality of cultured oysters in Japan and its prevention. USA–Japan Meeting on Aquaculture, Tokyo, Japan, pp 29–34
Lidie KB, Ryan JC, Barbier M, Van Dolah FM (2005) Gene expression in Florida red tide dinoflagellate Karenia brevis: analysis of an expressed sequence tag library and development of DNA microarray. Mar Biotechnol 5, 481–493
Martinez W, Martinez A (2002) Computational Statistics Handbook with MATLAB. (Boca Raton, FL: Chapman & Hall/CRC)
McKillen DJ, Chen YA, Chen C, Jenny MJ, Trent HF, Robalino J, McLean DC, Gross PS, Chapman RW, Warr GW, Almeida JS (2005) Marine genomics: a clearing-house for genomic and transcriptomic data of marine organisms. BMC Genomics 6, 34
Newell RIE (1988) Ecological changes in Chesapeake Bay: are they the result of over-harvesting the Eastern oyster (Crassostrea virginica)? In: Understanding the Estuary: Advances in Chesapeake Bay Research, Lynch MP, Krome EC, eds. (Solomons, MD: Chesapeake Research Consortium) pp 536–546
Peatman EJ, Wei X, Feng J, Liu L, Kucuktas H, Li P, He C, Rouse D, Wallace R, Dunham R, Liu Z (2004) Development of expressed sequence tags from Eastern oyster (Crassostrea virginica): lessons learned from previous efforts. Proc Mar Biotechnol 6, S491–S496
Renn SC, Aubin-Horth N, Hofmann HA (2004) Biologically meaningful expression profiling across species using heterologous hybridization to a cDNA microarray. BMC Genomics 5, 42
Robalino J, Bartlett T, Shepard E, Prior S, Jaramillo G, Scura E, Chapman RW, Gross PS, Browdy CL, Warr GW (2005) Double-stranded RNA induces sequence-specific antiviral silencing in addition to nonspecific immunity in a marine shrimp: convergence of RNA interference and innate immunity in the invertebrate antiviral response? J Virol 79, 13561–13571
Soletchnik P, Le Moine O, Faury N, Razet D, Geairon P, Goulletquer P (1999) Summer mortality of the oyster in the Bay Marennes-Oleron: spatial variability of environment and biology using a geographical information system (GIS). Aquat Living Res 12, 131–143
Storey JD (2002) A direct approach to false discovery rate. J R Statist Soc B 64, 479–498
Storey JD, Tibshirani R (2003) Statistical significance for genomewide studies. Proc Natl Acad Sci USA 100, 9440–9445
Tanguy A, Guo X, Ford, S (2004) Discovery of genes expressed in response to Perkinsus marinus challenge in eastern (Crassostrea virginica) and Pacific (C gigas) oysters. Gene 338, 121–131
Tanguy A, Boutet I, Laroche J, Moraga D (2005) Molecular identification and expression study of differentially regulated genes in the Pacific oyster Crassostrea gigas in response to pesticide exposure. FEBS J 272, 390–403
von Schalburg KR, Rise ML, Cooper GA, Brown GD, Gibbs AR, Nelson CC, Davidson WS, Koop BF (2005) Fish and chips: various methodologies demonstrate the utility of a 16,006-gene salmonid microarray. BMC Genomics 6, 126
Acknowledgments
The authors thank Dr. Fran van Dolah (NOAA) and Kristy B. Lidie for donation of the K. brevis chloroplast clones, Mara Lennard for the donation of the catfish Bob1 clone, and Dr. Javier Robalino for the donation of the white spot syndrome virus clones. This paper is Marine Genome Project Contribution no. 30 and no. 618 of the South Carolina Department of Natural Resources. Part of the research (graduate research support for M.J. Jenny) was conducted under an award from the Estuarine Reserves Division, Office of Ocean and Coastal Resource Management, National Ocean Service, National Oceanic and Atmospheric Administration and the Science to Achieve Results Graduate Fellowship, U.S. Environmental Protection Agency. Construction and characterization of the Crassostrea cDNA microarray was also supported by National Oceanic and Atmospheric Administration Center of Excellence for Oceans and Human Health at the Hollings Marine Laboratory (National Ocean Service, National Centers for Coastal Ocean Science), as well as the Ernest F. Hollings Visiting Scholar Program (C. Cunningham). Additional funding came from the Oyster Disease Research Program, NOAA agreement no. NA16RG1039 and Sea GrantProject R/SAQ-08-NSI. The authors would also like to thank Drs. A.F. Holland and P.A. Sandifer for their encouragement and enthusiasm throughout all phases of the work. We thank Drs. Christopher Bayne, Philippe Roch, and Andrew Mount for careful reading of the manuscript and many helpful suggestions. The oyster cDNA microarray is publicly available to the research community on a cost-recovery basis (contact Paul Gross; grossp@musc.edu).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jenny, M.J., Chapman, R.W., Mancia, A. et al. A cDNA Microarray for Crassostrea virginica and C. gigas . Mar Biotechnol 9, 577–591 (2007). https://doi.org/10.1007/s10126-007-9041-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10126-007-9041-1