Abstract
A novel TECTA mutation, p.R1890C, was found in a Dutch family with nonsyndromic autosomal dominant sensorineural hearing impairment. In early life, presumably congenital, hearing impairment occurred in the midfrequency range, amounting to about 40 dB at 1 kHz. Speech recognition was good with all phoneme recognition scores exceeding 90%. An intact horizontal vestibuloocular reflex was found in four tested patients. The missense mutation is located in the zona pellucida (ZP) domain of α-tectorin. Mutations affecting the ZP domain of α-tectorin are significantly associated with midfrequency hearing impairment. Substitutions affecting other amino acid residues than cysteines show a significant association with hearing impairment without progression. Indeed, in the present family progression seemed to be absent. In addition, the presently identified mutation affecting the ZP domain resulted in a substantially lesser degree of hearing impairment than was previously reported for DFNA8/12 traits with mutations affecting the ZP domain of α-tectorin.
Similar content being viewed by others
Introduction
Various phenotypes of autosomal dominant, sensorineural, nonsyndromic hearing impairment can be distinguished. Up until now, 54 loci have been mapped and 21 genes have been identified (Van Camp and Smith 2005). The different gene loci for these nonsyndromic types of hearing impairment have been designated DFN (DeaFNess) and are numbered in chronological order of discovery. Autosomal dominant types are referred to as DFNA. These types of hearing impairment can be characterized by age of onset, presence and degree of progression, severity of hearing impairment, and audiometric configuration. One of these configurations is the “cookie-bite,” U-shaped or trough-shaped audiometric configuration of midfrequency hearing impairment. This phenotype can be found in DFNA8/12 (TECTA) and DFNA13 (COL11A2) (Van Camp and Smith 2005).
DFNA8/12 has been mapped to the chromosomal locus 11q22–24 (Kirschhofer et al. 1998; Verhoeven et al. 1997) and is caused by mutations in TECTA (Verhoeven et al. 1998; Govaerts et al. 1998). This gene encodes α-tectorin, the most important noncollagenous component of the tectorial membrane in the cochlea and the otolith membrane in the maculae of the vestibular system. The tectorial membrane consists of an extracellular matrix overlying the organ of Corti that contacts the outer cochlear hair cells and plays an important role in intracochlear sound transmission by ensuring optimal cochlear feedback (Legan et al. 2000). Recently, Legan et al. (2005) described a second major role for the tectorial membrane. Neural tuning curves relating to inner hair cell stimulation showed a remarkable loss in sensitivity in mutant mice. This effect was attributed to a loss in coupling of the inner hair cell bundles to tectorial membrane motion caused by enlargement of the subtectorial space in the region of these hair cells.
In this study we report a Dutch family with nonprogressive, presumably prelingual, midfrequency hearing impairment caused by a novel missense mutation affecting the zona pellucida (ZP) domain of α-tectorin. Statistical testing was performed by using this mutation and the mutations previously identified to show the significance of the association between the type of mutation and the respective phenotype.
Patients and methods
A three-generation pedigree was established for the present family. Twenty-four family members participated in this study. Their medical history was taken and otologic examination was performed. Nonhereditary causes of hearing loss were excluded and written informed consent was obtained. All individuals included in this study underwent pure tone audiometry; speech audiometry was only performed in affected persons. Vestibular function was tested in four patients. Blood samples were obtained for linkage analysis from 11 affected and 13 unaffected family members.
Audiometric analysis
Pure tone and speech audiometry were performed in a sound treated room, conforming to the International Standards Organisation (ISO) standards (ISO 389 1985; ISO 8253-1 1989). The individual 95th percentile threshold values of presbyacusis (P 95) in relation to the patient's sex and age were derived for each frequency by using the ISO 7029 method (ISO 7029 1984). Individuals were considered affected if the best hearing ear showed thresholds beyond the P 95.
Speech audiometry
Speech audiometry was performed in a quiet environment using standard monosyllabic Dutch word lists. The maximum monaural phoneme score (% correct recognition) was derived from a performance vs. intensity plot.
Vestibuloocular examination
Vestibuloocular responses were evaluated in four patients (aged 23, 39, 43, and 45 years) by using electronystagmography (in the dark with open eyes) with computer analysis as previously described (Verhagen et al. 1992). The horizontal vestibuloocular reflex (VOR) was evaluated by performing velocity-step tests at 90°/s. Ocular motor evaluation comprised saccades, smooth pursuit eye movements, and optokinetic nystagmus, as well as gaze-evoked and spontaneous nystagmus.
Genotyping
DNA from lymphocytes was isolated as described by Miller et al. (1988). Polymorphic microsatellite markers were amplified by using 50 ng genomic DNA in 20 mM Tris–HCl (pH 8.4), 50 mM KCl, 1.5 mM MgCl2, 2U Taq Polymerase (Invitrogen, Breda, the Netherlands), 125 μmM dATP, 125 μmM dGTP, 125 μmM dTTP, 1.5 μmM dCTP, 400 nM 32P-dCTP (111TBq/mmol; MP Biomedicals, Irvine, CA, USA), 2.7 ng forward primer, and 2.7 ng reverse primer in a total volume of 25 μl. PCR products were analyzed as described by Kremer et al. (1994).
Linkage analysis
Two-point lod scores and the maximum lod score were calculated with the Mlink and Ilink subroutines, respectively, of the LINKAGE program (version 5.1) (Lathrop and Lalouel 1984; Lathrop et al. 1986). Full penetrance was assumed and disease allele frequency was set at 0.0001. The phenocopy rate was estimated to be 1 in 1000.
Mutation analysis
Primer sequences and conditions for amplification of all exons of TECTA (GenBank ID NM_005422.1) are available on request. PCR products were sequenced by using the ABI PRISM BigDye Terminator Cycle Sequencing V2.0 Ready Reaction Kit and analyzed with the ABI PRISM 3730 DNA analyzer (Applied Biosystems, Foster City, CA, USA). Of the last exon, 450 nucleotides of the 3′-untranslated region were analyzed. Segregation in the family and presence of c.248C > T nucleotide change in 165 controls was tested by amplification of exon 3 and subsequent digestion with NlaIII according to the manufacturer's protocols (Invitrogen). Segregation in the family and presence of the nucleotide substitution, c.5668C > T, in 184 controls was tested by amplification of exon 18 and subsequent digestion with BsgI according to the manufacturer's protocols (Invitrogen).
Data analysis
Binaural mean threshold levels were used to perform cross-sectional linear regression analysis (threshold on age) at each frequency. The presence/absence of progression was assessed by testing whether or not the 95% confidence interval for the regression coefficient (slope) included zero. Phoneme recognition scores (binaural mean) were plotted against the degree of impairment, expressed as the binaural mean pure tone average (PTA) at the frequencies 1, 2, and 4 kHz (PTA1,2,4 kHz). Curve fitting in this performance-impairment plot was conducted according to a previously reported method using a sigmoidal equation with variable slope (Bom et al. 2001). A previously described group of subjects with only presbyacusis (De Leenheer et al. 2002) was used as a reference group. Only those subjects with presbyacusis who had a matching degree of impairment by PTA1,2,4 kHz were used for statistical testing. A 2 × 2 contingency table was constructed by using one of the fitted curves as a dividing line for dichotomizing the speech recognition scores in both groups and Fisher's exact test was performed. Contingency tables (2 × 2) were also used to test for the possible significance (p < 0.05 in Fisher's exact test) of associations between genotype and phenotype features.
Results
The pedigree (Fig. 1) comprised three generations and included 14 affected family members (8 males and 6 females), 11 of whom were still alive and willing to participate in this study. The pattern of inheritance is autosomal dominant. There was no evidence of any other cause of hearing impairment, except for one patient who had undergone stapes replacing surgery for unilateral otosclerosis. This patient was included in this study by only using audiometric data from her other ear. The first symptoms of hearing impairment were reported at ages ranging from <1 to 30 years. Vestibular symptoms were not reported. Otoscopy was normal in all subjects except the patient mentioned above. Pure tone audiograms (Fig. 2) were symmetric and often showed a so-called cookie-bite shape, which indicates that predominantly the middle frequencies are affected. The highest threshold was most often found at 1kHz, followed by 2kHz. It was usually in the range of 40–60 dB, apparently independent of age.
Pedigree and chromosome 11 haplotypes of family W04-077. The at-risk haplotype is indicated by the black bar. If the phase is unknown, the haplotype is marked with a thin line. Brackets indicate deduced marker alleles. The marker order is according to the Human Genome Working Draft May 2004 and corresponds to the deCODE genetic map (Kong et al. 2002).
Binaural mean air conduction thresholds of the affected family members shown in audiogram format. Numbers designate age in years. Dotted line represents P50 thresholds for the last age >20 years according to ISO 7029 (ISO 7029 1984). The panels are ordered by age at last complete audiogram.
Audiometric analysis
All available cross-sectional data, combining individual longitudinal measurements (seven cases), i.e., only those pertaining to the patient's last complete audiogram, and single-snapshot measurements (four cases) are shown for the separate frequencies in Figure 3. We found only shallow positive or negative slopes. Indeed, the 95% confidence interval for the slope of the regression line included zero at all frequencies. Thus, it appeared that the thresholds did not depend on age. Age could therefore be ignored and mean thresholds covering all ages were calculated (Fig. 4).
Figure 4 includes the mean evaluable thresholds previously reported for other DFNA8/12 families with nonprogressive hearing loss (Kirschhofer et al. 1998; Govaerts et al. 1998; Iwasaki et al. 2002). Remarkably, the mean threshold for the present family is substantially lower than the mean threshold of any of the other families. The difference amounts to 20–30 dB. The previously described families showed a tendency for a maximum threshold at 2 kHz rather than 1 kHz in the present family.
Speech recognition
All of the present patients had maximum phoneme recognition scores of >90% at a PTA1,2,4 kHz of 60 dB or better (Fig. 5). The presbyacusis data used as a reference were matched by selecting the subjects with a PTA1,2,4kHz of 60 dB or better. Fischer's exact test indicated a significant difference in favor of the present patients (p = 0.018 or 0.034, depending on the dividing line). As can be read from the fitted curves, the difference amounted to about 10–15% on average at a PTA1,2,4kHz close to 60 dB (Fig. 5).
Binaural mean phoneme recognition score (% correct) plotted against binaural mean PTA1, 2, 4 kHz for patients of the present family (open circles and bold solid line) and for a group of subjects with presbyacusis showing a matching degree of impairment (crosses and thin solid line; Kremer et al. 1994).
Vestibuloocular examination
None of the patients had any vestibular symptoms. Ocular motor tests were normal in the four patients tested. Intact vestibular function was found with a normal horizontal VOR gain in all of these patients, with an abnormally long dominant VOR time constant in two of them.
Linkage analysis
The two DFNA loci associated with midfrequency hearing impairment, DFNA8/12 and DFNA13, were tested for linkage with polymorphic markers flanking or within the causative genes TECTA and COL11A2, respectively. For the DFNA8/12 locus, linkage was detected with a maximum lod score of 4.00 (θ = 0.0) for the intragenic marker D11S4167. The DFNA13 locus was excluded (lod score < –2.0) by using the markers D6S1666, D6S273, D6S276, and D6S1615. The at-risk haplotype, which includes the DFNA8/12 locus on chromosome 11, is depicted in Figure 1.
DNA sequencing of TECTA
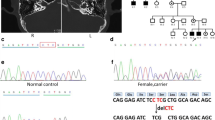
DFNA8/12 is caused by missense mutations in TECTA. We analyzed TECTA for mutations in all 23 exons and intron–exon boundaries in family member III-11. Both the forward and reversed DNA sequence of exon 3 showed a nucleotide change (c.248C > T) that is predicted to result in the substitution of a methionine for a threonine at position 83 (Fig. 6A). This novel variant removes an NlaIII restriction site. The substitution segregated with the disease and was not present in 330 alleles from healthy controls as determined by restriction analysis (data not shown). In addition, another change, c.5668C > T, was detected that is predicted to result in the substitution of a cysteine for an arginine at position 1890 in the ZP domain of α-tectorin (Fig. 6C). This change creates a BsgI restriction site, segregated with the disease and was not present in 368 alleles from healthy controls (data not shown). Finally, four known single nucleotide polymorphisms (rs612969, rs536069, rs520805, and rs586473; www.ncbi.nlm.nih.gov/SNP) were identified in family member III-11.
Nucleotide changes in exon 3 and exon 18 of TECTA. Chromatograms showing the c.248C>T transversion in exon 3 in individual III:11 (A), and the normal DNA sequence in a healthy individual (B). Chromatograms showing the c.5668C>T transversion in exon 18 individual III:11 (C), and the normal DNA sequence in a healthy individual (D).
Genotype–phenotype correlation
Alpha-Tectorin is composed of three distinct modules: the entactin G1 domain, the zonadhesin (ZA) domain with von Willebrand factor type D repeats, and the ZP domain (Legan et al. 1997). Mutations affecting the ZP domain are associated with midfrequency hearing impairment, whereas mutations in the ZA domain are associated with hearing impairment primarily affecting the high frequencies (Table 1) (Kirschhofer et al. 1998; Verhoeven et al. 1998; Govaerts et al. 1998; Iwasaki et al. 2002; Alloisio et al. 1999; Balciuniene et al. 1999; Pfister et al. 2004; Moreno Pelayo et al. 2001). Hearing impairment can range from mild to severe and has prelingual or postlingual onset. Furthermore, mutations causing substitution of cysteine residues are associated with progressive hearing impairment.
Two-by-two contingency tables were used for testing the possible significance (p < 0.05 in Fisher's exact test) of associations between the position and nature of the amino acid substitutions and the specific phenotypic features (Table 2).
Mutations in the ZP domain are significantly associated with midfrequency hearing impairment, whereas mutations in the ZA domain are associated with high-frequency hearing loss (p = 0.018). Cysteine replacing mutations are associated with progressive hearing impairment (p = 0.014).
Discussion
Two changes, p.R1890C and p.T83M, were detected in α-tectorin in a family with autosomal dominant midfrequency hearing impairment. The mutation p.R1890C is present in the ZP domain of α-tectorin, whereas p.T83M is not found in a specific domain. Both amino acid residues are conserved among human, rat, and chicken α-tectorin. The identity between these three orthologs is higher than 70%. Considering the phenotype and position of the mutation, p.R1890C is likely to be causative for hearing impairment in the family under study, because cysteines in the ZP domain are involved in the intra- and/or intermolecular interactions of α-tectorin, and mutations of cysteines or other amino acid residues into cysteines have been shown to cause autosomal dominant hearing impairment, due to problems with α-tectorin secretion (Jovine et al. 2002). Also, the p.T83M change seems to be a relatively mild amino acid substitution according to the Dayhoff table (Dayhoff et al. 1978). The nucleotide change is not predicted to affect splicing of the TECTA mRNA. However, because this change was not present in controls, it cannot be ruled out that it has an effect on the phenotype of these patients or it may even act synergistically with the p.R1890C mutation.
It has been suggested in a previous report that patients with TECTA-based midfrequency hearing impairment might be less prone to presbyacusis because they are generally exposed to lower-than-usual levels of sound energy at the level of the organ of Corti (Kirschhofer et al. 1998). Unaided phoneme recognition scores were all >90% and proved to be significantly higher than scores found in a reference group of patients with presbyacusis. The difference amounted to 10–15% at around a PTA1,2,4 kHz of 60 dB. It is likely that this difference in favor of the present patients relates to the fact that they have substantially better thresholds at higher frequencies than the subjects with presbyacusis. If a protective effect against presbyacusis indeed occurs, it might be less important in the present family, given the observation that the threshold levels at 4–8 kHz were about 20–30 dB, whereas they amounted to 40–60 dB in the previously reported families (Fig. 4). Nevertheless, we have attempted to substantiate the presence of any such protective effect by correcting the individual thresholds at each frequency for the median (P50) threshold indicated by ISO 7029 (ISO 7029 1984). Linear regression analysis (threshold on age) similar to the one illustrated in Figure 3 performed on the corrected thresholds produced plots with regression lines that mainly showed negative slopes. However, none of these slopes was significantly negative (data not shown) and thus did not support any hypotheses about protection from presbyacusis.
Differences in phenotype among DFNA8/12 families appear to be related to the position of the mutations in either the ZP or the ZA domain of α-tectorin as well as the nature of the amino acid substitution (Table 1). Mutations in the ZP and ZA domains have been related to mid- and high-frequency hearing impairment, respectively (Kirschhofer et al. 1998; Verhoeven et al. 1998; Govaerts et al. 1998; Iwasaki et al. 2002; Alloisio et al. 1999; Balciuniene et al. 1999; Pfister et al. 2004; Moreno Pelayo et al. 2001). Substitutions replacing cysteines have been implicated in progressive hearing impairment (Alloisio et al. 1999; Balciuniene et al. 1999; Pfister et al. 2004; Moreno Pelayo et al. 2001). There are now sufficient observations to show the significance or these genotype–phenotype correlations (Table 2A, B).
There is a significant association between mutations affecting the ZP domain and the midfrequency impairment phenotype as well as between mutations affecting the ZA domain and the high-frequency impairment phenotype (Table 2A). There is also a significant association between mutations causing cysteine-replacing substitutions and age-related progression of the hearing impairment (Table 2B). It should be noted that the level of significance (0.05 as usual) might be adjusted for multiple testing as two independent tests were performed with the same material. This would require the p value to be <0.0253 instead of <0.05. In Table 2, the p values fulfilled this requirement; however, the collective data prior to the inclusion of the present family (values in parenthesis in Table 2A, B) did not. Thus, the data on TECTA genotypes and the corresponding phenotypes support the previously suggested associations on the basis of significant test results.
In the four tested patients, we found an intact horizontal VOR. Goodyear and Richardson (2002) found abundant expression of α-tectorin in utricular and saccular otoconial membrane, not in the crista ampullaris. Legan et al. (2000) found substantially reduced otoconial membranes with only a few, abnormally large, scattered otoconia in mice homozygous for a targeted deletion in α-tectorin; the cupulae in the semicircular canals were normal. These mice did not show any spontaneous vestibular behavioral deficit, such as circling or head bobbing. However, no righting or swimming tests were performed. The present finding that the horizontal VOR was intact in the present patients conforms with the previous finding of Iwasaki et al. (2002), i.e., the only other study in which vestibular testing was performed. However, otolith reflexes are not covered by the type of vestibular testing performed. A suggestive anamnestic finding was reported for a French family whose three affected members had started walking only at 24 months of age (Alloisio et al. 1999). Vestibular testing was not performed in this family. It seems that the question as to whether DFNA8/12 patients have normal or abnormal otolith reflexes still requires appropriate instrumental otolith-testing paradigms. Given the type of histopathology observed in animal models for TECTA (Legan et al. 2000), imaging of the inner ear cannot be expected to contribute to the knowledge pool as yet. Indeed, no abnormal scanning results have been obtained up to now.
In summary, we identified a novel mutation, p.R1890C, in the ZP domain of α-tectorin in a family with a somewhat milder degree of midfrequency hearing impairment than previously reported to be associated with mutations in this domain. Statistical tests on genotype–phenotype correlations of the present and collective data on DFNA8/12 have now revealed a significant association between the affected α-tectorin domain (ZP or ZA) and the type of hearing impairment (mid- or high-frequency type), as well as a significant association between a cysteine-replacing substitution and age-related progression in hearing impairment.
References
Alloisio N, Morlé L, Bozon M et al. Mutation in the zonadhesin-like domain of α-tectorin associated with autosomal dominant non-syndromic hearing loss. Eur. J. Hum. Genet. 7:255–258, 1999.
Balciuniene J, Dahl N, Jalonen P et al. alpha-Tectorin involvement in hearing disabilities: one gene—two phenotypes. Hum. Genet. 105:211–216, 1999.
Bom SJH, De Leenheer EMR, Lemaire FX et al. Speech recognition scores related to age and degree of hearing impairment in DFNA2/KCNQ4 and DFNA9/COCH. Arch. Otolaryngol. Head Neck Surg. 127:1045–1048, 2001.
Dayhoff MO, Schwart RM, Orcutt BC. A model of evolutionary change in proteins. In: Dayhoff M (ed) Atlas of Protein Sequence and Structure, vol. 5. National Biomedical Research Foundation, Washington, DC, pp 345–352, 1978.
De Leenheer EMR, Huygen PLM, Coucke PJ et al. Longitudinal and cross-sectional phenotype analysis in a new, large Dutch DFNA2/KCNQ4 family. Ann. Otol. Rhinol. Laryngol. 111:267–274, 2002.
Goodyear RJ, Richardson GP. Extracellular matrices associated with the apical surfaces of sensory epithelia in the inner ear: molecular and structural diversity. J. Neurobiol. 52:212–227, 2002.
Govaerts PJ, De Ceulaer G, Daemers K et al. A new autosomal-dominant locus (DFNA12) is responsible for a non-syndromic, mid-frequency, prelingual and nonprogressive sensorineural hearing loss. Am. J. Otol. 19:718–723, 1998.
ISO 7029 Acoustics. Threshold as Hearing by Air Conduction as a Function of Age and Sex for Otologically Normal Persons. International Organisation for Standardization, Geneva, 1984.
ISO 389 Acoustics. Standard Reference Zero for the Calibration of Pure Tone Air Conduction Audiometers. International Organisation for Standardization, Geneva, 1985.
ISO 8253-1 Acoustics. Audiometric Test Methods, I: Basic Pure Tone Air and Bone Conduction Threshold Audiometry. International Organisation for Standardization, Geneva, .1989.
Iwasaki S, Harada D, Usami S et al. Association of clinical features with mutation of TECTA in a family with autosomal dominant hearing loss. Arch. Otolaryngol. Head Neck Surg. 128:913–917, 2002.
Jovine L, Qi H, Williams Z et al. The ZP domain is a conserved module for polymerization of extracellular proteins. Nat. Cell Biol. 4:457–461, 2002,
Kirschhofer K, Kenyon JB, Hoover DM et al. Autosomal-dominant, prelingual, nonprogressive sensorineural hearing loss: localization of the gene (DFNA8) to chromosome 11q by linkage in an Austrian family. Cytogenet. Cell Genet. 82: 126–130, 1998.
Kong A, Gudbjartsson DF, Sainz J et al. A high-resolution recombination map of the human genome. Nat. Genet. 31:241–247, 2002.
Kremer H, Pinckers A, van den Helm B et al. Localization of the gene for dominant cystoid macular dystrophy on chromosome 7p. Hum. Mol. Genet. 3:299–302, 1994.
Lathrop GM, Lalouel JM. Easy calculations of lod scores and genetic risks on small computers. Am. J. Hum. Genet. 36: 460–465.
Lathrop GM, Lalouel JM, White RL (1986) Construction of human linkage maps: likelihood calculations for multilocus linkage analysis. Genet. Epidemiol. 3:39–52, 1984.
Legan PK, Rau A, Keen JN et al. The mouse tectorins. Modular matrix proteins of the inner ear homologous to components of the sperm–egg adhesion system. J. Biol. Chem. 272: 8791–8801, 1997.
Legan PK, Lukashkina VA, Goodyear RJ et al. A targeted deletion in α-tectorin reveals that the tectorial membrane is required for the gain and timing of cochlear feedback. Neuron. 28:273–285, 2000.
Legan PK, Lukashkina VA, Goodyear RJ et al. A deafness mutation isolates a second role for the tectorial membrane in hearing. Nat. Neurosci. 8:1035–1042, 2005.
Mazzoli M, Van Camp G, Newton V et al. Recommendations for the description of genetic and audiological data for families with non-syndromic hereditary hearing impairment. Hereditary Hearing Loss Homepage (see http://webhost.ua.ac.be/hhh/) Accessed July 2005.
Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 16:1215, 1988.
Moreno Pelayo MA, del Castillo I, Villamar M et al. A cysteine substitution in the zona pellucida domain of α-tectorin results in autosomal dominant, postlingual, progressive, mid frequency hearing loss in a Spanish family. J. Med. Genet. 38:E13, 2001.
Pfister M, Thiele H, Van Camp G et al. A genotype–phenotype correlation with gender effect for hearing impairment caused by TECTA mutations. Cell. Physiol. Biochem. 14:369–376, 2004.
Van Camp G, Smith RJH. Hereditary Hearing Loss Homepage. World Wide Web URL: http://webhost.ua.ac.be/hhh/ Accessed July 2005.
Verhagen WIM, ter Bruggen JP, Huygen PLM, Oculomotor, auditory, and vestibular responses in myotonic dystrophy. Arch. Neurol. 49:954–996, 1992.
Verhoeven K, Van Camp G, Govaerts PJ et al. A gene for autosomal dominant nonsyndromic hearing loss (DFNA12) maps to chromosome 11q22–24. Am. J. Human. Genet. 60: 1168–1173, 1997.
Verhoeven K, Van Laer L, Kirschhofer K et al. Mutations in the human α-tectorin gene cause autosomal dominant non-syndromic hearing impairment. Nat. Genet. 19:60–62, 1998.
Acknowledgments
We wish to thank the participating family. This project was supported by the European Commission FP6 Integrated Project EUROHEAR, LSHG-CT-20054-512063. Arjan P. M. de Brouwer is supported by the Heinsius-Houbolt Foundation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Rutger F. Plantinga and Arjan P. M. de Brouwer contributed equally in this work.
Rights and permissions
About this article
Cite this article
Plantinga, R.F., de Brouwer, A.P.M., Huygen, P.L.M. et al. A Novel TECTA Mutation in a Dutch DFNA8/12 Family Confirms Genotype–Phenotype Correlation. JARO 7, 173–181 (2006). https://doi.org/10.1007/s10162-006-0033-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10162-006-0033-z