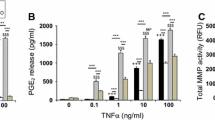
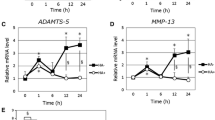
Abstract
Mechanical loading and the fibronectin fragments (FN-fs) are known to stimulate the anabolic and catabolic processes in articular cartilage, possible through pathways mediated by ·NO. This study examined the combined effects of dynamic compression and the NH2-hep I or COOH-hep II FN-fs on the expression levels of iNOS and COX-2 and production of ·NO and PGE2 release. Both types of fragments induced iNOS and COX-2 expression and stimulated the production of ·NO release. This response was inhibited by dynamic compression. Inhibitor experiments indicated that both dynamic compression and the iNOS inhibitor were important in restoring cell proliferation and proteoglycan synthesis in the presence of the FN-fs. This is the first study which demonstrates a downregulation of the FN-f-induced iNOS and COX-2 expression by dynamic compression. The combination of mechanical and pharmacological interventions makes this study a powerful tool to examine further the interactions of biomechanics and cell signalling in osteoarthritis.
Similar content being viewed by others
Abbreviations
- ·NO:
-
Nitric oxide
- PGE2 :
-
Prostaglandin E2
- iNOS:
-
Inducible nitric oxide synthase
- COX-2:
-
Cyclo-oxygenase
- OA:
-
Osteoarthritis
- FN-f:
-
Fibronectin fragment
References
Amin AR, Attur M, Patel RN, Thakker GD, Marshall PJ, Rediske J, Stuchin SA, Patel IR, Abramson SB: Superinduction of cyclooxygenase-2 activity in human osteoarthritis-affected cartilage. Influence of nitric oxide. J Clin Invest 99, 1231–1237 (1997)
Arner EC, Tortorella MD: Signal transduction through chondrocyte integrin receptors induces matrix metalloproteinase synthesis and synergizes with interleukin-1. Arthritis Rheum 38(9), 1304–1314 (1995)
Blain EJ: Mechanical regulation of MMPs. Front Biosci 12, 507–527 (2007)
Blain EJ, Mason DJ, Duance VC: The effect of cyclical compressive loading on gene expression in articular cartilage. Biorheology 40, 111–117 (2001)
Blom AB, van der Kraan PM, van den Berg WB: Cytokine targeting in osteoarthritis. Curr Drug Targets 8, 283–292 (2007)
Backlund MG, Mann JR, Holla VR, Buchanan FG, Tai HH, Musiek ES, Milne GL, Katkuri S, DuBois RN: 15-Hydroxyprostaglandin dehydrogenase is down-regulated in colorectal cancer. J Biol Chem 280(5), 3217–3223 (2005)
Chowdhury TT, Bader DL, Lee DA: Dynamic compression inhibits the synthesis of nitric oxide and PGE 2 by IL-1β stimulated chondrocytes cultured in agarose constructs. Biochem Biophys Res Commun 285, 1168–1174 (2001)
Chowdhury TT, Bader DL, Lee DA: Dynamic compression counteracts IL-1β induced release of nitric oxide and PGE 2 by superficial zone chondrocytes cultured in agarose constructs. Osteoarthr Cartil 11, 688–696 (2003a)
Chowdhury TT, Bader DL, Shelton JC, Lee DA: Temporal regulation of chondrocyte metabolism in agarose constructs subjected to dynamic compression. Arch Biochem Biophys 417, 105–111 (2003b)
Chowdhury TT, Bader DL, Lee DA: Dynamic compression counteracts IL-1beta induced iNOS and COX-2 activity by human chondrocytes cultured in agarose constructs. Biorheology 43, 413–429 (2006)
Chowdhury TT, Arghandawi S, Brand J, Akanji OO, Bader DL, Salter DM, Lee DA: Dynamic compression counteracts IL-1β induced inducible nitric oxide synthase and cyclo-oxygenase-2 expression in chondrocyte/agarose constructs. Arthritis Res Ther 10(2), R35 (2008)
Del Carlo M Jr, Loeser RF: Nitric oxide-mediated chondrocyte cell death requires the generation of additional reactive oxygen species. Arthritis Rheum 46(2), 394–403 (2002)
Del Carlo M, Schwartz D, Erickson EA, Loeser R: Endogenous production of reactive oxygen species is required for stimulation of human articular chondrocyte matrix metalloproteinase production by fibronectin fragments. Free Radical Biol Med 42, 1350– 1358 (2007)
De Rosa E, Urciuolo F, Borselli C, Gerbasio D, Imparato G, Netti PA: Time and space evolution of transport properties in agarose-chondrocyte constructs. Tissue Eng 12(8), 2193–2201 (2006)
Deschner J, Hofman CR, Piesco NP, Agarwal S: Signal transduction by mechanical strain in chondrocytes. Curr Opin Clin Nutr Metab Care 6, 289–293 (2003)
Forsyth CB, Pulai J, Loeser RF: Fibronectin fragments and blocking antibodies to alpha2beta1 and alpha5beta1 integrins stimulate mitogen-activated protein kinase signaling and increase collagenase 3 (matrix metalloproteinase 13) production by human articular chondrocytes. Arthritis Rheum 46(9), 2368–2376 (2002)
Gemba T, Valbracht J, Alsalmeh S, Lotz M: Focal adhesion kinase and mitogen-activated protein kinases are involved in chondrocyte activation by the 29-kDa amino-terminal fibronectin fragment. J Biol Chem 277(2), 907–911 (2002)
Griffin TM, Guilak F: The role of mechanical loading in the onset and progression of osteoarthritis. Exerc Sport Sci Rev 33(4), 195–200 (2005)
Guilak F, Fermor B, Keefe FJ, Kraus VB, Olson SA, Pisetsky DS, Setton LA, Weinberg JB: The role of biomechanics and inflammation in cartilage repair and injury. Clin Orthop Relat Res 423, 17–26 (2004)
Homandberg GA (1999) Potential regulation of cartilage metabolism in osteoarthritis by fibronectin fragments. In: Malemud CJ (ed) Special issue “fundamental pathways in osteoarthritis”. Front Biosci 4:D713–D730
Homandberg GA, Hui F: Association of proteoglycan degradation with catabolic cytokine and stromelysin release from cartilage cultured with fibronectin fragments. Arch Biochem Biophys 334(2), 325–331 (1996)
Homandberg GA, Meyers R, Xie D: Fibronectin fragments cause chondrolysis of bovine articular cartilage slices in culture. J Biol Chem 267(6), 3597–3604 (1992)
Homandberg GA, Hui F, Wen C, Purple C, Bewsey K, Koepp H, Huch K, Harris A: Fibronectin-fragment-induced cartilage chondrolysis is associated with release of catabolic cytokines. Biochem J 321(3), 751–757 (1997)
Homandberg GA, Wen C, Hui F: Cartilage damaging activities of the fibronectin fragments derived from cartilage and synovial fluid. Osteoarthr Cartil 6, 231–244 (1998)
Johnson A, Smith R, Saxne T, Hickery M, Heinegard D: Fibronectin fragments cause release and degradation of collagen-binding molecules from equine explant cultures. Osteoarthr Cartil 12, 149–159 (2003)
Jones KL, Brown M, Ali SY, Brown RA: An immunohistochemical study of fibronectin in human osteoarthritic and disease-free articular cartilage. Ann Rheum Dis 46, 809–815 (1987)
Leddy HA, Awad HA, Guilak F: Molecular diffusion in tissue-engineered cartilage constructs: effects of scaffold material, time, and culture conditions. J Biomed Mater Res B Appl Biomater 70(2), 397–406 (2004)
Lee DA, Bader DL: Compressive strains at physiological frequencies influence the metabolism of chondrocytes seeded in agarose. J Orthop Res 15, 181–188 (1997)
Lee DA, Knight MM: Mechanical loading of chondrocytes embedded in 3D constructs: in vitro methods for assessment of morphological and metabolic response to compressive strain. Methods Mol Med 100, 307–324 (2004)
Loeser RF: Molecular mechanisms of cartilage destruction: mechanics, inflammatory mediators, and aging collide. Arthritis Rheum 54(5), 1357–1360 (2006)
Loeser RF, Forsyth CB, Samarel AM, Im HJ: Fibronectin fragment activation of proline-rich tyrosine kinase PYK2 mediates integrin signals regulating collagenase-3 expression by human chondrocytes through a protein kinase C-dependent pathway. J Biol Chem 278(27), 24577–24585 (2003)
Lorenzo P, Bayliss MT, Heinegard D: Altered patterns and synthesis of extracellular matrix macromolecules in early OA. Matrix Biol 23, 381–391 (2004)
Lotz M: The role of nitric oxide in articular cartilage damage. Rheum Dis Clin North Am 25, 269–282 (1999)
Lust G, Burton-Wurster N, Leipold H: Fibronectin as a marker for OA. J Rheumatol 14, 28–29 (1987)
Madhavan S, Anghelina M, Rath-Deschner B, Wypasek E, John A, Deschner J, Piesco N, Agarwal S: Biomechanical signals exert sustained attenuation of proinflammatory gene induction in articular chondrocytes. Osteoarthr Cartil 14, 1023–1032 (2008)
Martin JA, Buckwalter JA: Effects of fibronectin on articular cartilage chondrocyte proteoglycan synthesis and response to IGF-1. J Orthop Res 16(6), 752–757 (1998)
Malemud CJ: Protein kinases in chondrocyte signalling and osteoarthritis. Clin Orthop Relat Res Suppl 427, 145–151 (2004)
Millward-Sadler SJ, Salter DM: Integrin-dependent signal cascades in chondrocyte mechanotransduction. Ann Biomed Eng 32, 435–446 (2004)
Pfaffl MW, Horgan GW, Dempfle L: Relative expression software tool (REST) for group wise comparison and statistical analysis of relative expression results in real time PCR. Nucleic Acids Res 30, e3 (2002)
Pichika R, Homandberg GA: Fibronectin fragments elevate nitric oxide (NO) and inducible NO synthetase (iNOS) levels in bovine cartilage and iNOS inhibitors block fibronectin fragment mediated damage and promote repair. Inflamm Res 53(8), 405–412 (2004)
Ragan PM, Badger AM, Cook M, Chin VI, Gowen M, Grodzinsky AJ, Lark MW: Down-regulation of chondrocyte aggrecan and type-II collagen gene expression correlates with increases in static compression magnitude and duration. J Orthop Res 17, 836–842 (1999)
Rao J, Otto WR: Fluorimetric DNA assay for cell growth estimation. Anal Biochem 207, 186–192 (1991)
Saklatvala J: Inflammatory signaling in cartilage: MAPK and NF-kappaB pathways in chondrocytes and the use of inhibitors for research into pathogenesis and therapy of osteoarthritis. Curr Drug Targets 8(2), 305–313 (2007)
Scher JU, Pillinger MH, Abramson SB: Nitric oxide synthases and osteoarthritis. Curr Rheum Reports 9, 9–15 (2007)
Stanton H, Ung L, Fosang AJ: The 45 kDa collagen-binding fragment o fibronectin induces MMP-13 synthesis by chondrocytes and aggrecan degradation by aggrecanases. Biochem J 364, 181–190 (2002)
Valhmu WB, Stazzone EJ, Bachrach NM, Saed-Nejad F, Fischer SG, Mow VC, Ratcliffe A: Load-controlled compression of articular cartilage induces a transient stimulation of aggrecan gene expression. Arch Biochem Biophys 353, 29–36 (1998)
Weinberg JB, Fermor B, Guilak F: NOS and COX interactions in cartilage and meniscus: relationships to joint physiology, arthritis and tissue repair. Subcell Biochem 42, 31–62 (2007)
Xie D, Hua F, Homandberg GA: Fibronectin fragments alter matrix protein synthesis in cartilage tissue cultured in vitro. Arch Biophys 307(1), 110–118 (1993)
Xie D, Hui F, Homandberg GA: Cartilage chondrolysis by fibronectin fragments is associated with release of several proteinases: stromelysin plays a major role in chondrolysis. Arch Biochem Biophys 307(1), 110–118 (1994)
Yasuda T: Cartilage destruction by matrix degradation products. Mod Rheumatol 16, 197–205 (2006)
Yasuda T, Nakamura T: Inhibition of nuclear factor-κB by hyaluronan in rheumatoid chondrocytes stimulated with COOH-terminal heparin-binding fibronectin fragment. Mod Rheumatol 17(5), 391–397 (2007)
Yasuda T, Poole AR: A fibronectin fragment induces type II collagen degradation by collagenase through an interleukin-1-mediated pathway. Arthritis Rheum 46, 138–148 (2002)
Yasuda T, Poole RA, Shimizu M, Nakagawa T, Julovi SM, Tamamura H, Fujii N, Nakamura T: Involvement of CD44 in induction of matrix metalloproteinases by a COOH-terminal heparin-binding fragment of fibronectin in human articular cartilage in culture. Arthritis Rheum 48(5), 1271–1280 (2003)
Yasuda T, Kakinuma T, Julovi SM, Yoshida M, Hiramitsu T, Akiyoshi M, Nakamura T: COOH-terminal heparin-binding fibronectin fragment induces nitric oxide production in rheumatoid cartilage through CD44. Rheumatology 43, 1116–1120 (2004)
Wolf A, Raiss RX, Steinmeyer J: FN metabolism of cartilage explants in response to the frequency of intermittent loading. J Orthop Res 21, 1081–1089 (2003)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Raveenthiran, S.P., Chowdhury, T.T. Dynamic compression inhibits fibronectin fragment induced iNOS and COX-2 expression in chondrocyte/agarose constructs. Biomech Model Mechanobiol 8, 273–283 (2009). https://doi.org/10.1007/s10237-008-0134-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10237-008-0134-1