Abstract
Wildlife diseases are in fashion. This is creating an explosion of related knowledge. Despite this, the dynamics of both wildlife and diseases and the changes in livestock and wildlife management make it increasingly difficult to overview the current situation of wildlife diseases in Europe. This paper aims to discuss the available management possibilities and to highlight current research priorities. One area that causes severe concern to authorities is diseases largely under control in domestic populations but still existing as a reservoir in wildlife. Multihost situations are also of concern for wildlife management and conservation, as diseases can affect the productivity and density of wildlife populations with an economic or recreational value. Concern about emerging diseases is rising in recent years, and these may well occur at the fertile livestock–wildlife interface. Wildlife-related zoonoses are a diverse and complex issue that requires a close collaboration between wildlife ecologists, veterinarians and public health professionals. A few risk factors can be identified in most of the relevant wildlife diseases. Among them are (1) the introduction of diseases through movements or translocations of wild or domestic animals, (2) the consequences of wildlife overabundance, (3) the risks of open air livestock breeding, (4) vector expansion and (5) the expansion or introduction of hosts. Wildlife disease control requires the integration of veterinary, ecology and wildlife management expertise. In addition to surveillance, attempts to control wildlife diseases or to avoid disease transmission between wildlife and livestock have been based on setting up barriers, culling, hygienic measures, habitat management, vector control, treatments and vaccination. Surveillance and descriptive studies are still valuable in regions, species or diseases that have received less attention or are (at least apparently) emerging. Nonetheless, limiting the research effort to the mere reporting of wildlife disease outbreaks is of limited value if management recommendations are not given at the same time. Thus, more experimental approaches are needed to produce substantial knowledge that enables authorities to make targeted management recommendations. This requires policy makers to be more aware of the value of science and to provide extra-funding for the establishment of multidisciplinary scientific teams.
Similar content being viewed by others
Introduction
Wildlife diseases are in fashion. This was recently highlighted by the H5N1 avian flu crisis, which has put the role of wildlife diseases worldwide on the front-pages of the newspapers (Anonymous 2006). This and other recent zoonotic events (West Nile fever in North America, severe acute respiratory syndrome, etc.) have greatly increased the general public’s interest in wildlife disease issues, and as a result, game managers, conservationists and governmental agencies have increased attention to wildlife disease surveillance and control. This contrasts with the virtual ignorance of this subject only a few years ago (Simpson 2002). The increased interest fuelled by mass media risks to reduce the problem to science fiction. By contrast, however, it is a complex situation that requires intense social and scientific debate (Angulo and Cooke 2002; Artois 2003). In the last two decades, ecologists, forest engineers and other professionals have joined veterinarians to form multidisciplinary research teams. This has triggered a scientific debate with an explosion of wildlife disease-related knowledge as evidenced by the increase in scientific papers published in peer-reviewed journals (Fig. 1). It has also led to a new approach to understanding disease agents in relation to the environment, and with a view to parasite host interactions, a whole new field grouped under the synonym “disease ecology” (Hudson et al. 2001).
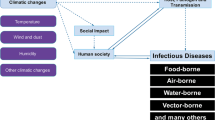
Among the most intriguing aspects of this new scientific branch is the link between wildlife pathogens, environment and human activities. This web of factors creates a dynamic situation where new pathogens or new hosts emerge, changes in population density or host behaviour affects disease prevalence and disease agents can suddenly boost their virulence and widen their host range. In this wide spectrum of interactions exists yet another complicating factor due to the increased risk of contact between wildlife, livestock and humans. This is because, on one hand, animal welfare politics and consumer requirements are moving the animal breeding industry from more intensive to more extensive farming systems, and on the other hand, wildlife populations are increasingly managed through feeding, translocations and even fencing, thus becoming more and more like extensively raised livestock with limited sanitary care (Fig. 2). These situations increase the exchange of pathogens or vectors (e.g. Laddomada et al. 1994; Gortázar et al. 2006). In addition, human activities have an influence on endangered and unmanaged wildlife, as the loss of certain habitats or food resources lead different species to exploit alternative resources (e.g. Iberian lynx feeding on tuberculosis (TB)-infected carrion, Perez et al. 2001; storks and kites foraging on rubbish dumps, Tortosa et al. 2002; Blanco et al. 2006). This creates another interface for human/livestock pathogens to become established and sustained in new hosts.
Some pathogens do exclusively infect a single host species. These pathogens are frequently specialised, highly coevolved parasites with limited effect on the primary host’s population (Crawley 1992; Vicente et al. 2004a, b), or the possible secondary hosts are just unknown. These are generally, in the absence of environmental changes, considered less relevant from the wildlife management and conservation and domestic animal perspective.
Many parasites, especially if environmental changes occur, can infect multiple host species and these are primarily responsible for emerging infectious disease outbreaks in humans, livestock and wildlife (Woolhouse 2002). Moreover, the ecological and evolutionary factors that constrain or facilitate such emergences are poorly understood. Fenton and Pedersen (2005) proposed a conceptual framework based on the pathogen’s between- and within-species transmission rates to describe possible configurations of a multihost-pathogen community that may lead to disease emergence. Spill over and apparent multihost situations are those where, without between-species transmission, the disease would not persist in the target host. In true multihost situations, the pathogen can independently persist in either host population in the absence of the other.
Along with the mentioned growth in wildlife research and knowledge, the dynamics of both wildlife and diseases and the changes in livestock and wildlife management make it increasingly difficult to overview the current situation of wildlife diseases. This paper reviews some of the most relevant wildlife diseases from a European perspective, aiming to discuss the available disease management possibilities and to highlight current research priorities.
The wildlife–domestic animal interface
This issue has been reviewed by Frölich et al. (2002) and Simpson (2002). A non-exhaustive list of relevant multihost situations regarding European wildlife is shown in Table 1. From the veterinary health perspective, true multihost situations in diseases that are notifiable, eradicated or almost under control in domestics are the worst, because a single spill over from wildlife to livestock may have severe consequences not only on health, but also on economy. Examples include bovine TB (Phillips et al. 2003) and avian influenza (Alexander 2000). Multihost situations are of less concern if the disease is not yet under control in domestic animals, as for example porcine circovirus type 2 (Vicente et al. 2004a, b) or toxoplasmosis (Gauss et al. 2006), and if adequate vaccines or treatments are available and extensively used in domestics, as for example in the case of porcine parvovirus and erysipelas (Ritzmann et al. 2000).
Sometimes problems arise when wildlife and domestic animals share disease agents whose antibodies cross-react with relevant diseases. For example, antibodies against pestiviruses other than classical swine fever may be found in pigs and in wild boar (Langedijk et al. 2001), causing alarm in animal health authorities.
Despite increasing knowledge, in many cases, the available information is still not sufficient to decide if a given “disease–wildlife species–livestock” triangle is of concern for animal health authorities or for wildlife managers (Simpson 2002). This may be the case, for example, for bluetongue, a vector-borne viral disease recently emerging in Europe that affects domestic and wild ruminants, and even of well-known cattle viral diseases, such as infectious bovine rhinotracheitis or other bovine viral diseases, where the actual role of deer still needs further clarification (Frölich et al. 2002). The recent foot-and-mouth disease outbreak in the UK showed that deer, at least at the densities existing in the UK, are not a true reservoir for this disease, as culling of infected livestock resolved the problem, but what would have happened if wild boar were abundant?
Parasitic diseases shared between wildlife and domestic animals have been reviewed by Simpson (2002). None of them alone is as relevant, as some of the viral diseases, but all together, cause huge economic losses, and wildlife may complicate attempts of parasite control in domestics. For example, Neospora caninum infection is present in wildlife, especially in red deer. This has important implications in the prevalence of infection in cattle farms (Almeria et al. 2007).
In summary, wildlife reservoirs need to be considered in the management of domestic animal health, and more scientific knowledge is strongly needed due to the changing nature of environment, disease agents and hosts.
Diseases affecting wildlife management and conservation
Multihost situations are also of concern for wildlife management and conservation, as diseases can be a threat for endangered species or affect the productivity and density of wildlife populations with an economic or recreational value (Gortázar et al. 2006). Viral diseases of birds that are shared between domestic flocks and wild birds can affect economically relevant game birds (e.g. avian pox and red-legged partridge, Buenestado et al. 2004), or endangered birds, often boosting vaccination of captive individuals (e.g. West Nile encephalitis, Siegal-Willott et al. 2006). Domestic and wild pigeons carry Trichomonas gallinae, a protozoan parasite that can be transmitted to their predators, including endangered raptors, especially when other traditional prey items become scarce (Real et al. 2000; Hofle et al. 2004). For example, it has been shown in wood pigeons that the parasites reduce body condition even in clinically healthy infested animals and thus makes them more susceptible to predation (Villanúa et al. 2006a, b). Other parasites can be transported by game bird releases from farms to the field (Millan et al. 2004a, b) and eventually affect endangered bird species. For example, this has been suggested in the case of a typical partridge capillarid nematode (Eucoleus contortus) found in a little bustard (Villanúa et al. 2007a, b). Anthelminthic treatment is not 100% effective (Villanúa et al. 2007a, b). The stress associated to the release of game birds, along with the end of any antiparasitic treatments, increases parasite excretion of these birds after release, increasing the transmission probability to wild birds (Villanúa et al. 2006a, b).
Two viral diseases of lagomorphs, myxomatosis since the 1950s and rabbit haemorrhagic disease since the 1990s, have caused a severe decline in rabbit numbers throughout continental Western Europe. Among the causes of the problem are translocations of domestic rabbits and voluntary or involuntary transmission of the virus to wild rabbits (Angulo and Cooke 2002). The combined effects of these diseases, probably exacerbated by hunting, have contributed largely to the adverse situation of many rabbit predators, such as the Iberian lynx and many raptors (Angulo and Villafuerte 2004; Williams et al. 2007). This is probably one of the best examples of the huge effect that shared wildlife diseases can have on conservation and on management.
Among carnivores, diseases shared between livestock and wildlife can cause mortality of valuable endangered species. Metapopulation theory suggests that dispersed and reduced populations of endangered wildlife are more prone to extinction through stochastical events, such as disease outbreaks (Macdonald 1993). This means that even apparent multihost situations or occasional spillovers may affect the conservation of rare species. For example, Aujeszky’s disease, a viral disease of swine and wild boar that can affect most mammals except man and primates, has been proposed as a risk for carnivore conservation (e.g. Banks et al. 1999; Vicente et al. 2005). Several Iberian lynx have died due to bovine TB in their last two strongholds in southern Spain (e.g. Perez et al. 2001). Consumption of infected prey or infected carcass remains is suspected as the way of transmission. Canine distemper in felids is an example for the potentially devastating effect of a pathogen in new host species (e.g. Vanmoll et al. 1995). It is diagnosed frequently in European wildlife (Baumgartner et al. 2003) and considered a serious concern for the conservation of Iberian lynx. Along with wildlife reservoirs such as the red fox, domestic dogs probably contribute to the risk of endangered wildlife getting in contact with the virus (Frölich et al. 2000). The same may occur with several other viral diseases of carnivores, but most situations are still insufficiently known. Disease monitoring is therefore an important element of recovery plans for rare species that are potential victims of epizootic pathogens (Macdonald 1993).
Among ungulates, two diseases shared between wild caprine (such as chamois and ibex) and domestic sheep and goats have important consequences on wildlife numbers and animal welfare. One is keratoconjunctivitis due to Mycoplasma conjunctivae in alpine chamois (Giacometti et al. 2002), and the second is sarcoptic mange (Sarcoptes scabiei), affecting several populations of mountain ungulates in Europe (e.g. Rossi et al. 2007). In both cases, the disease is suspected to spread from domestic livestock to wildlife and is responsible for repeated outbreaks that affect hunting harvest and population dynamics.
Even disease control measures can affect conservation and animal welfare, as shown by the badger culling debate in relation with TB control (Delahay 2006; Karesh et al. 2005) and by the adverse effect of fox den gasing, a measure to control rabies, on badger populations (Woodroffe et al. 2005). Culling of wild or captive endangered birds is a risk in the current avian flu crisis (Webster et al. 2006; Olsen et al. 2006).
Finally, disease transmission between wildlife and livestock can undermine conservation efforts if wildlife is seen as the source of a disease affecting livestock or human health (Brook and McLachlan 2006).
Wildlife-related emerging diseases
Potential emerging infectious diseases are those where the pathogen will become self-sustaining in the new host once the initial (environment, host- or pathogen-related) barrier to infection has been crossed. Concern about emerging diseases is rising in recent years, and these may well occur at the fertile livestock–wildlife interface (Cunningham 2005). Wild animals are the most likely source of new emerging infectious diseases that put at risk the health of human beings and livestock (Anonymous 2004).
Chronic wasting disease, for example, a transmissible spongiform encephalopathy of North American cervids, could become an emerging disease in Europe if the geographical barrier is crossed (for example, importing diseased deer from North America). Prion diseases are not a real problem in European wildlife, but testing is ongoing (Schettler et al. 2006). Another example of a potential emerging disease is Rift Valley fever, a mosquito-borne zoonotic viral disease leading to serious economic losses in livestock, particularly sheep, in Africa. Global climatic change, vector expansion or movements of domestic animals may eventually bring this disease to Europe. In addition, a number of flaviviruses that exist in tropical and subtropical America may eventually be imported through travellers or translocated animals. The host diversity of these viruses in their native range (De Thoisy et al. 2004), along with the current expansion of vectors such as Aedes albopictus in the Mediterranean (Mitchell 1995), may eventually cause outbreaks in Europe.
Recent cases of wildlife-related emerging diseases include the HP avian flu crisis, affecting waterfowl and other bird species in different continents, including Europe (Chen et al. 2005; Kwon et al. 2005; Brown et al. 2006), West Nile virus in Eastern and Mediterranean Europe (Bakonyi et al. 2006), and leishmaniosis, which has spread into several new areas in Europe (Ferroglio et al. 2006).
Other than viral diseases, mycobacterial infections constitute frequent emerging or re-emerging disease agents in wildlife. Bovine TB due to Mycobacterium tuberculosis complex is increasingly important in wild ungulates, especially the wild boar, in Mediterranean Europe (Bollo et al. 2000; Vicente et al. 2006), but see also Machackova et al. 2003 for central and eastern Europe), and paratuberculosis (M. avium paratuberculosis) is now considered highly prevalent among wild rabbits and other wildlife in Scotland (Daniels et al. 2003), red deer in the Italian Alps (Fraquelli et al. 2005) and other wildlife elsewhere. In the case of Mediterranean wild boar populations, increasingly artificial management, such as fencing, feeding, watering and translocations, has been suggested as a possible cause for TB re-emergence (Vicente et al. 2007). In both cases, re-emergence of these mycobacterial diseases constitutes a severe barrier to eradication in livestock.
A new pestivirus, close to the border disease virus cluster, has recently been found in Pyrenean chamois, Rupicapra pyrenaica (Arnal et al. 2004, Frölich et al. 2005), and is apparently linked with important mortalities in the eastern third of these mountains. Research is going on to investigate the association of this pestivirus with disease in Pyrenean chamois and to reveal the source of this disease emergence (Hurtado et al. 2004). Sarcoptic mange, another disease of wild caprine, is present in many European mountain habitats and continues to expand to new areas (e.g. Rossi et al. 2007). Other strains of S. scabiei do affect carnivores (Gortázar et al. 1998), wild boar (Kutzer 1986) and humans (Green 1989). In contrast, cases in deer are extremely rare. Despite this, a yearly increasing number of red deer die due to mange in northern Spain, in an area where chamois mange is endemic. For example, two cases of roe deer mange have been found in the same region in 2006. This suggests a possible disease emergence in new hosts that is currently under investigation.
In summary, actual or potential emerging diseases deserve attention, including the study of the underlying causes for the emergence of infectious diseases, which are often related to anthropogenic and environmental changes (Kuiken et al. 2003; Cunningham 2005).
Wildlife-related zoonoses
Rabies is the most classical wildlife-related zoonosis. In Europe, the red fox is the main reservoir of this viral disease. Rabid foxes can transmit the virus to wild and domestic mammals and humans or infect pets or livestock that can in turn infect humans. After causing serious concern in the second half of the twentieth century, rabies control was almost achieved through intense oral vaccination campaigns of foxes (Artois et al. 2001). This was the first sound success of wildlife vaccination as a disease control measure and proved to be preferable to the traditional methods based on fox population reduction. Despite this success, rabies appears to be re-emerging in some parts of Europe. This can be explained by a relaxation of vaccination campaigns in apparently rabies-free regions, an increase in fox densities and the expansion of new rabies hosts such as the racoon dog, Nictereutes procyonoides (Holmala and Kauhala 2006). In addition, bat rabies has been recorded in several European countries, even with a few fatal human cases (Calisher et al. 2006).
Bovine TB is mainly a disease of domestic cattle and goats, but can affect many other domestic and wild species, as well as humans. The existence of wildlife TB reservoirs is the main limiting factor for controlling this disease in livestock (Phillips et al. 2003). Rabies and bovine TB share several characteristics. Both are worldwide important zoonoses that cause significant mortality in developing countries. In Europe, human mortality due to these diseases is very limited due to the existing measures to control them in their animal hosts (dog and fox vaccination in rabies, testing and culling of infected livestock in TB) and to the existence of general preventive public health measures. Control measures in wildlife are quite expensive and even unpopular and make these diseases still relevant despite their limited impact on human health. in addition, at least in the case of bovine TB, some under-reporting of cases may exist. For example, most M. tuberculosis complex strains of caprine and bovine type, isolated from wild ungulates in southern Spain, have also been identified in humans (Gortázar et al. 2005), but in too many occasions these may be diagnosed as human TB if molecular typing is not performed.
In other, more recent (or recently detected) zoonoses, such as HP avian flu and, to a lesser extent, West Nile and other flaviviruses, Hanta virus or E-hepatitis, the role of wildlife in the epidemiology is still not completely understood and urgently deserves more research. In any case, wildlife has a relevant role in the spreading of diseases and as disease carriers over large distances and between countries, as is evident in the case of wild animal movements (Smith et al. 2006; Calisher et al. 2006).
Tularaemia is an example of a disease that, similarly to rabies, is mainly maintained in wildlife populations (e.g. invertebrates, rodents, hares), with occasional spill over to other animals and to humans. As eradication seems impossible, surveillance and hygienic preventive measures are needed to avoid outbreaks. One example occurred in 1998 with the Iberian hares (Lepus granatensis) of Spain (Puertas et al. 1999). On that occasion, the existence of a tissue bank with frozen spleen samples allowed to reveal the presence of the disease agent (Francisella tularensis) in hares shot several years before the 1998 outbreak. Wildlife monitoring data showed that the disease emergence was apparently linked to a sudden increase in Iberian hare numbers, rather than to the importation of infected European brown hares (L. europaeus), as suggested (Artois 2003). This example shows how relevant it is not only to set up wildlife disease surveillance schemes, but also to bank tissues. It also underlines the need to combine disease surveillance with the monitoring of wildlife abundance and the study of wildlife ecology (Gortázar et al. 2007).
Salmonella species are infectious agents causing numerous cases of human illness and important losses to the livestock industry each year. The two most common serovars, S. enteritidis and S. typhimurium, are well known to have different animals as reservoir for the disease in Europe: poultry in the former and pigs and cattle in the latter. In Europe, there are large differences in salmonellosis prevalence (De Jong and Ekdahl 2006). Wildlife is infected with these serovars through exposure to human or livestock residues and then transport the agents back to the farms. Wildlife can also host a variety of other salmonella serovars that are of zoonotic interest (Millan et al. 2004a, b).
Along with the nematodes of the genus Trichinella, the cestodes Echinococcus granulosus and E. multilocularis are the parasitic helminths of greatest zoonotic concern. E. granulosus not only has a domestic dog–sheep (and other livestock) cycle, but also a sylvatic wolf–wild ruminant (and other herbivores) one. These cycles may get linked through dogs consuming carcass remains of hunted game and wolves consuming livestock as carrion or as prey (e.g. Sobrino et al. 2006). E. multilocularis has no domestic cycle, and human cases occur through contact with contaminated fox faeces in endemic areas. In recent years, increases in the urban fox population have been observed in many countries of the northern hemisphere. As a result, E. multilocularis has entered the urban environment. Controlled baiting with the anthelminthic praziquantel showed that a pronounced reduction in E. multilocularis egg contamination is feasible in urban areas where the organism is highly endemic (Hegglin et al. 2003).
Finally, tick-transmitted diseases are among the most frequent wildlife-related zoonoses in the northern hemisphere. The agents are varied and include tick-borne encephalitis virus, Anaplasma phagocitophylum (the causative agent of human granulocytic ehrlichiosis), Lyme disease and several others. In most of these, wildlife hosts play a role either as disease reservoirs or by increasing the tick numbers (Lindgren et al. 2000; De la Fuente et al. 2005).
In summary, wildlife-related zoonoses are a diverse and complex issue that requires a close collaboration between wildlife ecologists, veterinarians and public health professionals.
Identifying the risks
A few general risk factors can be identified in most of the wildlife diseases listed in Table 1. Among them, the most frequent one is the introduction of diseases through movements or translocations of wild or domestic animals. Examples include HP avian flu (poultry and exotic bird trade and waterfowl movements), myxomatosis (voluntary disease translocation) and food-and-mouth disease in the UK (involuntary disease translocation). The European continent, for historical reasons, has been a source of disease spread across the world (for example, bovine TB, a European cattle disease nowadays present worldwide). In turn, introduction of exotic species into Europe also conveys important risks of introduction of previously inexistent diseases, especially when foreign species range in or share habitats with native susceptible species, leading to situations where the native species become endangered (McInnes et al. 2006). For example, this is the case of the North American wapiti (carrying the trematode Fascioloides magna, highly pathogenic in the red deer, Novobilsky et al. 2006), the grey squirrel (carrying a pox virus highly pathogenic in the red squirrel, McInnes et al. 2006) or the American mink (carrying viral diseases that can threaten native mustelids, Yamaguchi and Macdonald 2001). Exotic wildlife is introduced for recreational hunting (wapiti, Sylvilagus, Barbary sheep), as a pet (water turtles) or garden animal (grey squirrel), for direct economic exploitation (American mink) or by accident (birds).
Overabundance of wildlife is a second relevant risk factor for wildlife diseases. European examples include TB (Vicente el al. 2007) and classical swine fever (Rossi et al. 2005) in wild boar and salmonellosis (Pennycott 1998) and trichomoniasis (Höfle et al. 2004) in relation with wildlife feeding. The assessment of overabundance and the available management tools have been discussed recently (Gortázar et al. 2006).
Open air farming also constitutes a risk situation. Some alternative livestock species are reared more extensively and thus are more prone to share diseases with wildlife, such as salmonellosis (e.g. Pennycott 1998). This includes farmed deer, game birds, ducks and geese. In recent years, animal welfare concerns have also promoted an increasing extensification of traditionally indoor-raised poultry and swine, increasing the transmission risks of diseases, such as HP avian influenza (Fouchier et al. 2004) or Aujeszky’s disease and swine brucellosis (Ruiz-Fons et al. 2006).
The expansion or introduction of vectors, including the consequences of global change, can benefit certain diseases (Mitchell 1995). For example, tick species are expanding northwards (Lindgren et al. 2000), and bluetongue vectors, such as Culicoides imicola, are found in new locations and in growing abundances in Mediterranean Europe, creating the conditions for the expansion of this viral disease of ruminants (Purse et al. 2005).
Finally, the expansion or introduction of hosts has been linked to disease risks in several occasions. This was already mentioned in the case of the racoon dog and rabies, but may also apply for cases such as the introduced grey squirrel (Duff et al. 1996) and the newly established (escaped) wild boar populations in the UK (Williams and Wilkinson 1998).
Risk situations are frequently linked to human effects on wildlife abundance and distribution. Hence, diseases must be considered in any wildlife management programme with the same degree of relevance as factors such as habitat or genetics.
The control of disease in wildlife populations
This subject has been reviewed by Wobeser (1994 and 2002) and Artois (2001 and 2003). In addition to surveillance, three basic forms of disease management strategies for wildlife are known: prevention of introduction of disease, control of existing disease or almost impossible eradication (Wobeser 2002). In any option, integration of veterinary, ecology and wildlife management expertise through multidisciplinary teams is strongly recommended (Artois 2003).
Wildlife disease control begins with surveillance, knowing which diseases are present, their past and current distribution and the trends in their prevalence. In Europe, wildlife disease surveillance is addressed by a series of regional, national and international schemes. Currently, parts of Europe benefit from wildlife surveillance efforts (frequently limited to a few diseases), while in other parts, no surveillance is done at all. Proper implementation of a complete surveillance effort must be a priority of the veterinary authorities, as it is accepted that those countries that conduct disease surveillance of their wild animal populations are more likely to detect the presence of infectious and zoonotic diseases and to swiftly adopt countermeasures (Morner et al. 2002).
Among the preventive actions, the most important one is by restricting translocation of wild animals to prevent movement of disease (Wobeser 2002). This includes the movement and release of farm-bred “wildlife”, an increasingly popular game management tool that needs a careful sanitary control (Fernandez de Mera et al. 2003). In this field, a more intense collaboration between governmental or supragovernmental agencies devoted to animal health and to wildlife management is needed. But even the movements of domestic animals can easily cause the introduction of new diseases or new vectors (e.g. rabbit haemorrhagic disease introduced from China into Germany, Angulo and Cooke 2002).
Attempts to control disease in wildlife populations, or to avoid disease transmission between wildlife and livestock, have been based on a variety of methods. These include setting up barriers, improving hygienic measures, culling, habitat management and feeding bans, vector control, treatments and vaccination (Wobeser 2002; Artois 2003; Karesh et al. 2005; Gortázar et al. 2006).
Setting up barriers to prevent wildlife contact with domestic livestock, or vice versa, is rather infrequent, and its use is limited to certain risk situations. For example, it may make sense to avoid badgers on cattle farms in TB endemic areas (Garnett et al. 2002) or to prevent wild boar contact with open air bred pigs or other livestock (Parra et al. 2005).
Hygienic measures, such as correct disposal of the hunting carcasses and carcass remains, should become compulsory in every country and for every hunting modality (Almeria et al. 2007). However, research to assess the true risks of such management in every particular situation is needed in favour of the conservation of some wildlife species that usually survive very fragmentally and at the edge of their possibilities in Europe.
Wildlife culling is almost never an effective means of controlling a wildlife-related disease. Moreover, it is a subject of intense scientific and social debate (e.g. badger culling for TB control, Donnelly et al. 2006). Only in the case of island populations, when geographical barriers limit animal dispersal or in the case of introduced species (pest species, where legal and social constraints to culling are minimal) or to cope with a point-source wildlife disease outbreak (centering culling on the disease focus, plus an outer ring of vaccination), is culling and eradication an option. By contrast, population reduction is a goal in many disease control efforts. This is a temporary measure, except if habitat modification is used to reduce host density more permanently or to alter host distribution or exposure to disease agents (Wobeser 2002; Acevedo et al. 2007; Gortázar et al. 2006). Selective culling is limited to situations in which affected individuals are readily identifiable (Wobeser 2002). This has been used in several attempts to control mange in wild ungulates, but obviously with little success, as not all individuals with visible lesions are detected, and not all infected individuals show visible lesions. For example, chamois mange spread in the Cantabrian Mountains (Spain) could not be controlled by culling visibly infected individuals (Fernandez-Moran et al. 1997, and unpublished reports). Social structure disruption with increased movement and, therefore, increased contact rate (at intra- or interspecific level, Donnelly et al. 2006) may be counterproductive consequences of depopulation, which could be followed by rapid recovery of population size, even with increased population turnover through compensatory reproduction (e.g. wild boar and swine fever after high hunting pressure, Guberti et al. 1998). Ultimately, the best management choice must run in parallel with intense campaigns that convince and inform the society (farmers, hunters, the general public, etc).
Habitat management to cope with diseases may have opposite goals. For example, feeding bans can reduce the habitat carrying capacity for ungulates and eventually reduce population density and aggregation, two key factors in infectious disease transmission (Acevedo et al. 2007; Vicente et al. 2007). The identification and correction of overabundance situations are key actions in the control of many infectious diseases (Gortázar et al. 2006). By contrast, it can be useful to improve rabbit habitat (even with supplementary feeding) to reach higher population density and improve herd immunity against diseases such as rabbit haemorrhagic disease (Forrester et al. 2003).
Vector control has sometimes proven helpful in field experiments (e.g. with rabbit fleas and myxomatosis, Trout et al. 1992), but vector diversity and other factors may limit the effectiveness of this management in more complex environments (Osacar et al. 2001).
Treatment of wildlife is increasingly frequent, especially against parasites in economically valuable game species. For example, Spanish ibex (Capra pyrenaica) have been treated against sarcoptic mange with ivermectin (Leon Vizcaino et al. 2001), and wood pigeons (Columba palumbus) have been treated against trichomonas with dimetridazole (Hofle et al. 2004). Anthelminthic treatments are frequent in ungulates and in game birds (Fernandez de Mera et al. 2004; Rodriguez et al. 2006; Villanúa et al. 2007a, b). In many cases, however, the actual effectiveness of these treatments is unclear, and ethical and public health issues need to be addressed. For example, the use of antibiotics in game species may affect meat hygiene. Exceptions include the already mentioned treatment of foxes against E. multilocularis (Hegglin et al. 2003).
Wildlife vaccination is exceptional, and it is normally limited to the most relevant diseases (those that cause serious economic losses, are almost under control in domestics and where wildlife reservoirs are paramount). In Europe, this is the case of fox rabies (Artois et al. 1993), classical swine fever (Kaden et al. 2002) and, probably soon, bovine TB. Vaccination of rabbits against myxomatosis and haemorrhagic disease with a recombinant live vaccine has risen concerns among the scientific community (Angulo and Cooke 2002). In contrast to culling, oral vaccination has the advantages of being painless, thus avoiding animal welfare problems, and does not cause behavioural problems such as increased dispersal or immigration. Vaccination makes sense if the huge investment is the only way to control a disease in its wildlife reservoir, if the costs are clearly outbalanced by the costs of remaining passive and provided that the effectiveness and safety of the vaccine have been tested in captivity. In most cases, vaccination needs to be combined with other management measures, and the ecology of the host species needs to be considered carefully (Table 2).
In summary, management of diseases of wild animals usually requires a change in human activities (Wobeser 2002), and a sound scientific basis is strongly needed before suggesting any corrective measures that can create or increase conflicts between the different stakeholders: veterinary authorities, hunters, conservationists, livestock breeders and the general public (Fig. 3). Moreover, the success of any wildlife disease management action must be assessed critically, including an analysis of the costs, of the ecological consequences and of the animal and human health and welfare benefits.
European priorities in wildlife disease research
Top ranking wildlife diseases to research on are those where (1) wildlife has a high probability of substantially affecting regional disease status, and (2) the disease has a strong impact on human health, economy, wildlife management and conservation (Fig. 4).
Relevance of wildlife diseases in Europe. From top to base diseases where wildlife is known to affect disease control chances, diseases where wildlife is suspected to be relevant, diseases mainly affecting wildlife populations and others with limited relevance but of interest for basic knowledge on disease ecology
Among the diseases listed in Table 1, not all have the same economic impact than others, and only in a few of them is disease control in wildlife reservoirs paramount for the success of the general control schemes. These diseases are the most critical ones, and European research efforts are clearly biased towards them: classical swine fever, rabies, bovine TB. Research on these diseases has helped to make important management decisions and, in some cases, has opened the way to oral vaccination of wildlife. Moreover, these examples, along with the more recent HP avian flu, have contributed to the view that wildlife diseases are relevant and that multidisciplinary science can contribute to the improvement of animal and human health. Nonetheless, even more research is needed in the remaining diseases, where current knowledge is limited regarding their management.
In a number of cases, scientific evidence is currently inadequate to determine if European wildlife has a high probability of substantially affecting regional disease status, or not. This is the case, for example, of bluetongue, bovine viral diarrhoea, alphaherpesvirus infections, malignant catarrhal fever, brucellosis (B. abortus and B. melitensis) and leishmaniosis. These diseases deserve a greater research effort, to determine their relevance in relation to wildlife.
Finally, many other diseases have either a more limited impact on economy and public health, or the role of wildlife in their control is not considered relevant. This is the case, for example, of many parasitic diseases. Research on these subjects exists, in part, because macroparasites are easy to handle in experimental studies, and it provides a valuable source of basic scientific knowledge on disease ecology, which may be applied later to more relevant diseases (e.g. Hudson et al. 2001; Vicente et al. 2004a, b).
Regarding the kind of research needed in the wildlife disease field, surveillance and descriptive studies, in general, are still valuable, especially in regions, species or diseases that have received less attention. Nonetheless, limiting the effort to the mere reporting of wildlife disease outbreaks is of limited value if management recommendations are not given at the same time. Moreover, the animal health authorities and the public may perceive wildlife veterinarians as purveyors of bad news (“you got this disease”) with no positive counterpart (“you can do this”). Therefore, more experimental approaches are strongly needed if the aim is to produce substantial knowledge that enables researchers to make targeted management recommendations.
Experimental studies should ideally combine indoor experiments, such as experimental infections or vaccination trials, with field experiments testing hypotheses regarding for example the effects of host aggregation and density on disease prevalence (e.g. Donnelly et al. 2006; Acevedo et al. 2007) as well as pathogen persistence. Again, a strong ecological background is needed. Mathematical modelling may help to identify those knowledge gaps that most urgently need experimental research (Morgan et al. 2006). Research effort must also be focused on the development of diagnostic tools appropriate for wildlife species (Simpson 2002). The main drawback is that experimental research requires more funding than surveillance alone, and the former needs to be carried out by multidisciplinary scientific teams, which are scarce.
Conclusion
Three actions can help to improve our knowledge on wildlife diseases and our capacity to deal with their consequences on animal and human health as well as conservation: (1) extend surveillance schemes to the not yet included regions and taxa and improve coordination between surveillance schemes and other wildlife monitoring, (2) promote experimental and multidisciplinary research on the relevant wildlife diseases and (3) include knowledge on wildlife diseases in the curricula of the European veterinary students.
References
Acevedo P, Vicente J, Höfle U, Cassinello J, Ruiz-Fons F, Gortazar C (2007) Estimation of European wild boar relative abundance and aggregation: a novel method in epidemiological risk assessment. Epidemiol Infect 135:519–527. DOI 10.1017/S0950268806007059
Alexander DJ (2000) A review of avian influenza in different bird species. Vet Microbiol 74:3–13
Almeria S, Vidal D, Ferrer D, Pabon M, Fernandez-de-Mera I, Ruiz-Fons F, Alzaga V, Marco I, Calvete C, Lavin S, Gortazar C, Lopez-Gatius F, Dubey JP (2007) Seroprevalence of Neospora caninum in non-carnivorous wildlife from Spain. Vet Parasitol 143:21–28
Angulo E, Cooke B (2002) First synthesize new viruses then regulate their release? The case of the wild rabbit. Mol Ecol 11:2703–2709
Angulo E, Villafuerte R (2004) Modelling hunting strategies for the conservation of wild rabbit populations. Biol Conserv 115:291–301
Anonymous (2004) The Lancet infectious diseases—getting out into the field, and forest. Lancet Infect Dis 4:127
Anonymous (2006) Influenza—bird flu moves west, spreading alarm. Science 311:1084
Arnal MC, Fernandez-de-Luco D, Riba L, Maley M, Gilray J, Willoughby K, Vilcek S, Nettleton PF (2004) A novel pestivirus associated with deaths in Pyrenean chamois (Rupicapra pyrenaica pyrenaica). J Gen Virol 85:3653–3657
Artois M (2003) Wildlife infectious disease control in Europe. J Mt Ecol 7:89–97
Artois M, Masson E, Barrat J, Aubert MFA (1993) Efficacy of 3 oral rabies vaccine-baits in the red fox—a comparison. Vet Microbiol 38:167–172
Artois M, Delahay R, Guberti V, Cheeseman C (2001) Control of infectious diseases of wildlife in Europe. Vet J 162:141–1452
Bakonyi T, Ivanics T, Erdelyi K, Ursu K, Ferenczi E, Weissenbock H, Nowotny N (2006) Lineage 1 and 2 strains of encephalitic West Nile virus, central Europe. Emerg Infect Dis 12:618–623
Banks M, Torraca LSM, Greenwood AG, Taylor DC (1999) Aujeszky’s disease in captive bears. Vet Rec 145:362–365
Baumgartner W, Alldinger S, Beineke A, Groters S, Herden C, Kaim U, Muller G, Seeliger F, Van Moll P, Wohlsein P (2003) Canine distemper virus—an agent looking for new hosts. Dtsch Tierarztl Wochenschr 110:137–142
Blanco G, Lemus JA, Grande J (2006) Faecal bacteria associated with different diets of wintering red kites: influence of livestock carcass dumps in microflora alteration and pathogen acquisition. J Appl Ecol 43:990–998
Bollo E, Ferroglio E, Dini V, Mignone W, Biolatti B, Rossi L (2000) Detection of Mycobacterium tuberculosis complex in lymphnodes of wild boar (Sus scrofa) by a target-amplified test system. J Vet Med B 47:337–342
Brook RK, McLachlan SM (2006) Factors influencing farmers’ concerns regarding bovine tuberculosis in wildlife and livestock around Riding Mountain National Park. J Environ Manage 80:156–166
Brown IH, Banks J, Manvell, RJ, Essen SC, Shell W, Slomka M, Londt B, Alexander DJ (2006) Recent epidemiology and ecology of influenza A viruses in avian species in Europe and the Middle East. Dev Biol 124:45–50
Buenestado F, Gortazar C, Millan J, Hofle U, Villafuerte R (2004) Descriptive study of an avian pox outbreak in wild red-legged partridges (Alectoris rufa) in Spain. Epidemiol Infect 132:369–374
Calisher CH, Childs JE, Field HE, Holmes KV, Schountz T (2006) Bats: important reservoir hosts of emerging viruses. Clin Microbiol Reviews 19:531–545
Chen H, Smith GJD, Zhang SY, Quin K, Wang J, Li KS, Webster RG, Peiris JSM, Guan Y (2005) H5N1 outbreak in migratory waterfowl. Nature 436:191–192
Crawley MJ (1992) Natural enemies. The population biology of predators, parasites and diseases. Blackwell, London
Cunningham AA (2005) A walk on the wild side—emerging wildlife diseases. They increasingly threaten human and animal health. BMJ 331:1214–1215
Daniels MJ, Henderson D, Greig A, Stevenson K, Sharp JM, Hutchings MR (2003) The potential role of wild rabbits Oryctolagus cuniculus in the epidemiology of paratuberculosis in domestic ruminants. Epidemiol Infect 130:553–559
De Jong B, Ekdahl K (2006) The comparative burden of salmonellosis in the European Union member states, associated and candidate countries. BMC Public Health 6:4
De la Fuente J, Naranjo V, Ruiz-Fons F, Höfle U, Fernández de Mera IG, Villanúa D, Almazán C, Torina A, Caracappa S, Kocan KM, Gortázar C (2005) Potential vertebrate reservoir hosts and invertebrate vectors of Anaplasma marginale and A. phagocytophilum in central Spain. Vector Borne Zoonotic Dis 5:390–401
Delahay RJ (2006) Badgers and bovine tuberculosis: the ecological complexities of managing a wildlife disease reservoir. Cattle Pract 14:7–11
De Thoisy B, Dussart P, Kazanji M (2004) Wild terrestrial rainforest mammals as potential reservoirs for flaviviruses (yellow fever, dengue 2 and St Louis encephalitis viruses) in French Guiana. Trans R Soc Trop Med Hyg 98:409–412
Donnelly CA, Woodroffe R, Cox DR, Bourne FJ, Cheeseman CL, Clifton-Hadley RS, Wei G, Gettinby G, Gilks P, Jenkins H, Johnston WT, Le Fevre AM, McInerney JP, Morrison WI (2006) Positive and negative effects of widespread badger culling on tuberculosis in cattle. Nature 439:843–846
Duff JP, Scott A, Keymer IF (1996) Parapox virus infection of the grey squirrel. Vet Rec 138:527
Fenton A, Pedersen AB (2005) Community epidemiology framework for classifying disease threats. Emerg Infect Dis 11:1815–1821
Fernandez de Mera IG, Gortazar C, Vicente J, Höfle U, Fierro Y (2003) Wild boar helminths: risks in animal translocations. Vet Parasitol 115:335–341
Fernandez de Mera IG, Vicente J, Gortazar C, Höfle U, Fierro Y (2004) Efficacy of an in-feed preparation of ivermectin against helminths in the European wild boar. Parasitol Res 92:133–136
Fernandez-Moran J, Gomez S, Ballesteros F, Quiros P, Benito JL, Feliu C, Nieto JM (1997) Epizootiology of sarcoptic mange in a population of Cantabrian chamois (Rupicapra pyrenaica parva) in Northwestern Spain. Vet Parasitol 73:163–171
Ferroglio E, Romano A, Trisciuoglio A, Poggi M, Ghiggi E, Sacchi P, Biglino A (2006) Characterization of Leishmania infantum strains in blood samples from infected dogs and humans by PCR-RFLP. Trans R Soc Trop Med Hyg 100:636–641
Forrester NL, Boag B, Moss SR, Turner SL, Trout RC, White PJ, Hudson PJ, Gould EA (2003) Long-term survival of New Zealand rabbit haemorrhagic disease virus RNA in wild rabbits, revealed by RT-PCR and phylogenetic analysis. J Gen Virol 84:3079–3086
Fouchier RAM, Schneeberger PM, Rozendaal FW, Broekman JM, Kemink SAG, Munstert V, Kuiken T, Rimmelzwaan GF, Schutten M, van Doornum GJJ, Koch G, Bosman A, Koopmans M, Osterhaus ADME (2004) Avian influenza A virus (H7N7) associated with human conjunctivitis and a fatal case of acute respiratory distress syndrome. Proc Natl Acad Sci 101:1356–1361
Fraquelli C, Carpi G, Bregoli M, Ostanello F, Pasolli C, Pozzato N, Rosati S (2005) Epidemiology of paratuberculosis in two red deer (Cervus elaphus) populations of Trentino (Northern Italy). Proceedings of the 8th International Colloquium on Paratuberculosis, Copenhagen Denmark, pp 605–612
Frölich K, Czupalla O, Haas L, Hentschke J, Dedek J, Fickel J (2000) Epizootiological investigations of canine distemper virus in free-ranging carnivores from Germany. Vet Microbiol 74:283–292
Frölich K, Thiede S, Kozikowski T, Jakob W (2002) A review of mutual transmission of important infectious diseases between livestock and wildlife in Europe. Ann N Y Acad Sci 969:4–13
Frölich K, Jung KS, Ludwig A, Liekfeld D, Gibert P, Gauthier D, Hars J (2005) Detection of a newly described pestivirus of Pyrenean chamois in France. J Wildl Diseases 41:606–610
Garnett BT, Delahay RJ, Roper TJ (2002) Use of cattle farm resources by badgers (Meles meles) and risk of bovine tuberculosis (Mycobacterium bovis) transmission to cattle. Proc R Soc Lond B Biol Sci 269:1487–1491
Gauss CBL, Dubey JP, Vidal D, Cabezon O, Ruiz-Fons F, Vicente J, Marco I, Lavin S, Gortazar C, Almeria S (2006) Prevalence of Toxoplasma gondii antibodies in red deer (Cervus elaphus) and other wild ruminants from Spain. Vet Parasitol 136:193–200
Giacometti M, Janovsky M, Belloy L, Frey J (2002) Infectious keratoconjunctivitis of ibex, chamois and other Caprinae. Rev Sci Tech Off Int Epizoot 21:335–345
Gortázar C, Villafuerte R, Blanco JC, Luco DF (1998) Enzootic sarcoptic mange in red foxes in Spain. Z Jagdwiss 44:251–256
Gortázar C, Vicente J, Samper S, Garrido J, Fernández-de-Mera IG, Gavín P, Juste RA, Martín C, Acevedo P, de la Puente M, Höfle U (2005) Molecular characterization of Mycobacterium tuberculosis complex isolates from wild ungulates in south-central Spain. Vet Res 36:43–52
Gortázar C, Acevedo P, Ruiz-Fons F, Vicente J (2006) Disease risks and overabundance of game species. E J Wildl Res 52:81–87
Gortázar C, Millán J, Acevedo P, Escudero MA, Marco J, Luco DF (2007) A large-scale survey of brown hare Lepus europaeus and Iberian hare L. granatensis populations at the limit of their range. Wildl Biol (in press)
Green MS (1989) Epidemiology of scabies. Epidemiol Rev 11:126–150
Guberti V, Rutili D, Ferrari G, Patta C, Oggaino A (1998) Estimate the threshold abundance for the persistence of the classical swine fever in the wild boar population of the eastern Sardinia. Report on measures to control classical swine fever in European wild boar. Document VI/7196/98-AL. Commission of the European Communities, Directorate General VI for Agriculture. Perugia, Italy
Hegglin D, Ward PI, Deplazes P (2003) Anthelmintic baiting of foxes against urban contamination with Echinococcus multilocularis. Emerg Infect Dis 9(10):1266–1272
Hofle U, Gortazar C, Ortiz JA, Knispel B, Kaleta EF (2004) Outbreak of trichomoniasis in a woodpigeon (Columba palumbus) wintering roost. E J Wildl Res 50(2):73–77
Holmala K, Kauhala K (2006) Ecology of wildlife rabies in Europe. Mamm Rev 36:17–36
Hudson PJ, Rizzoli A, Grenfell BT, Heesterbeek H, Dobson AP (2001) The ecology of wildlife diseases. Oxford Univ. Press, Oxford
Hurtado A, Aduriz G, Gomez N, Oporto B, Juste RA, Lavin S, Lopez-Olvera JR, Marco I (2004) Molecular identification of a new pestivirus associated with increased mortality in the Pyrenean chamois (Rupicapra pyrenaica pyrenaica) in Spain. J Wildl Dis 40:796–800
Kaden V, Heyne H, Kiupel H, Letz W, Kern B, Lemmer U, Gossger K, Rothe A, Bohme H, Tyrpe P (2002) Oral immunisation of wild boar against classical swine fever: concluding analysis of the recent field trials in Germany. Berl Munch Tierarztl Wochenschr 115(5–6):179–185
Karesh WB, Cook RA, Bennett EL, Newcomb J (2005) Wildlife trade and global disease emergence. Emerg Infect Dis 11:1000–1002
Kuiken T, Fouchier R, Rimmelzwaan G, Osterhaus A (2003) Emerging viral infections in a rapidly changing world. Curr Opin Biotechnol 14(6):641–646
Kutzer E (1986) On the treatment of the sarcoptic mange at wild boar and pig with ivermectin (ivomec). Deut Tierarzt Wschr 93(9):426–429
Kwon YK, Joh SJ, Kim MC, Lee YJ, Choi JG, Wee SH, Sung HW, Kwon JH, Kang MI, Kim JH (2005) Highly pathogenic avian influenza in magpies (Pica pica serica) in South Korea. J Wildl Dis 41:618–623
Laddomada A, Patta C, Oggiano A, Caccia A, Ruiu A, Cossu P, Firinu A (1994) Epidemiology of classical swine fever in Sardinia—a serological survey of wild boar and comparison with African swine fever. Vet Rec 134(8):183–187
Langedijk JPM, Middel WGJ, Meloen RH, Kramps JA, de Smit JA (2001) Enzyme-linked immunosorbent assay using a virus type-specific peptide based on a subdomain of envelope protein E-rns for serologic diagnosis of pestivirus infections in swine. J Clin Microbiol 39(3):906–912
Leon-Vizcaino L, Cubero MJ, Gonzalez-Capitel E, Simon MA, Perez L, de Ybanez MRR, Ortiz JM, Candela MG, Alonso F (2001) Experimental ivermectin treatment of sarcoptic mange and establishment of a mange-free population of Spanish ibex. J Wildl Dis 37(4):775–785
Lesellier S, Palmer S, Dalley DJ, Dave D, Johnson L, Hewinson RG, Chambers MA (2006) The safety and immunogenicity of Bacillus Calmette–Guerin (BCG) vaccine in European badgers (Meles meles). Vet Immunol Immunopathol 112(1–2):24–37
Lindgren E, Talleklint L, Polfeldt T (2000) Impact of climatic change on the northern latitude limit and population density of the disease-transmitting European tick Ixodes ricinus. Environ Health Perspect 108(2):119–123
Macdonald DW (1993) Rabies and wildlife—a conservation problem. Onderstepoort J Vet Res 60(4):351–355
Machackova M, Matlova L, Lamka J, Smolik J, Melicharek I, Hanzlikova M, Docekal J, Cvetnic Z, Nagy G, Lipiec M, Ocepek M, Pavlik I (2003) Wild boar (Sus scrofa) as a possible vector of mycobacterial infections: reviews of literature and critical analysis of data from Central Europe between 1983 to 2001. Vet Med 48:51–65
McInnes CJ, Wood AR, Thomas K, Sainsbury AW, Gurnell J, Dein FJ, Nettleton PF (2006) Genomic characterization of a novel poxvirus contributing to the decline of the red squirrel (Sciurus vulgaris) in the UK. J Gen Virol 87:2115–2125
Millan J, Aduriz G, Moreno B, Juste RA, Barral M (2004a) Salmonella isolates from wild birds and mammals in the Basque Country (Spain). Rev Sci Tech Off Int Epizoot 23(3):905–911
Millan J, Gortazar C, Villafuerte R (2004b) A comparison of the helminth faunas of wild and farm-reared red-legged partridge. J Wildl Manage 68(3):701–707
Mitchell CJ (1995) Geographic spread of Aedes-albopictus and potential for involvement in arbovirus cycles in the Mediterranean Basin. J Vector Ecol 20(1):44–58
Morgan ER, Lundervold M, Medley GF, Shaikenov BS, Torgerson P, Milner-Gulland EJ (2006) Assessing risks of disease transmission between wildlife and livestock: the Saiga antelope as a case study. Biol Conserv 131:244–254
Morner T, Obendorf DL, Artois M, Woodford MH (2002) Surveillance and monitoring of wildlife diseases. Rev Sci Tech Off Int Epizoot 21:67–76
Novobilsky A, Horackova E, Hirtova L, Modry D, Koudela B (2006) The giant liver fluke Fascioloides magna (Bassi 1875) in cervids in the Czech Republic and potential of its spreading to Germany. Parasitol Res 100:549–553
Olsen B, Munster VJ, Wallensten A, Waldenström J, Osterhaus ADME, Fouchier RAM (2006) Global patterns of influenza A virus in wild birds. Science 312:384–388
Osacar JJ, Lucientes J, Calvete C, Peribanez MA, Gracia MJ, Castillo JA (2001) Seasonal abundance of fleas (Siphonaptera: Pulicidae, Ceratophyllidae) on wild rabbits in a semiarid area of northeastern Spain. J Med Entomol 38(3):405–410
Osterhaus ADME, Uytdehaage FGCM, Visser IKG, Vedder EJ, Reijnders PJH, Kuiper J, Brugge HN (1988) Seal vaccination success. Nature 337:21
Parra A, Larrasa J, Garcia A, Alonso JM, de Mendoza JH (2005) Molecular epidemiology of bovine tuberculosis in wild animals in Spain: a first approach to risk factor analysis. Vet Microbiol 110(3–4):293–300
Pennycott TW (1998) Population density and infectious disease at bird tables. Vet Rec 142:523
Perez J, Calzada J, Leon-Vizcaino L, Cubero MJ, Velarde J, Mozos E (2001) Tuberculosis in an Iberian lynx (Lynx pardina). Vet Rec 148(13):414–415
Phillips CJ, Foster CR, Morris PA, Teverson R (2003) The transmission of Mycobacterium bovis infection to cattle. Res Vet Sci 74:1–15
Puertas CA, Baruque MLM, Lobo IB, Megido MJG, Cuadrado CR, Arenas LAS (1999) Epidemic outbreak of tularemia in Palencia. Rev Clin Esp 199(11):711–715
Purse BV, Mellor PS, Rogers DJ, Samuel AR, Mertens PPC, Baylis M (2005) Climate change and the recent emergence of bluetongue in Europe. Nat Rev Microbiol 3(2):171–181
Real J, Manosa S, Munoz E (2000) Trichomoniasis in a Bonelli’s eagle population in Spain. J Wildl Dis 36:64–70
Ritzmann M, Gyra H, Johannes S, Hausleithner D, Heinritzi K (2000) Comparative investigations of a combined vaccine against porcine parvovirus and swine erysipelas and corresponding monovalent vaccines in different vaccination schedules. Tierarztl Pax 28(1):23–27
Rodriguez O, Fernandez de Mera IG, Vicente J, Peña A, Gortazar C (2006) Efficacy of in-feed-administered ivermectin on Elaphostrongylus cervi first-stage excretion in red deer (Cervus elaphus). Parasitol Res 98:176–178
Rossi S, Fromont E, Pontier D, Cruciere C, Hars J, Barrat J, Pacholek X, Artois M (2005) Incidence and persistence of classical swine fever in free-ranging wild boar (Sus scrofa). Epidemiol Infect 133(3):559–568
Rossi L, Fraquelli C, Vesco U, Permunian R, Sommavilla GM, Carmignola G, Da Pozzo R, Meneguz PG (2007) Descriptive epidemiology of a scabies epidemic in chamois in the Dolomite Alps, Italy. Eur J Wildl Res 53(2):131–141. DOI 10.1007/s10344-006-0067-x
Ruiz-Fons F, Vicente J, Vidal D, Höfle U, Villanúa D, Gauss C, Segales J, Almeria A, Montoro V, Gortazar C (2006) Seroprevalence of six reproductive pathogens in European wild boar (Sus scrofa) from Spain: the effect on wild boar female reproductive performance. Theriogenology 65:731–743
Schettler E, Steinbach F, Eschenbacher-Kaps I, Gerst K, Meussdoerffer F, Risch K, Streich WJ, Frolich K (2006) Surveillance for prion disease in cervids, Germany. Emerg Infect Dis 12(2):319–322
Siegal-Willott JL, Carpenter JW, Glaser AL (2006) Lack of detectable antibody response in greater flamingos (Phoenicopterus ruber ruber) after vaccination against West Nile virus with a killed equine vaccine. J Avian Med Surg 20(2):89–93
Simpson VR (2002) Wild animals as reservoirs of infectious diseases in the UK. Vet J 163:128–146
Smith RP, Rand PW, Lacombe EH, Morris SR, Holmes DW, Caporale DA (2006) Role of bird migration in the long-distance dispersal of Ixodes dammini, the vector of Lyme disease. J Infect Dis 174(1):221–224
Sobrino R, Gonzalez LM, Vicente J, Fernández de Luco D, Garate T, Gortázar C (2006) Echinococcus granulosus (Cestoda, Taeniidae) in the Iberian wolf. Parasitol Res 99:753–756
Tortosa FS, Caballero JM, Reyes-Lopez J (2002) Effect of rubbish dumps on breeding success in the white stork in southern Spain. Waterbirds 5:39–43
Trout RC, Ross J, Tittensor AM, Fox AP (1992) The effect on a British wild rabbit-population (Oryctolagus cuniculus) of manipulating myxomatosis. J Appl Ecol 29(3):679–686
Vanmoll P, Alldinger S, Baumgartner W, Adami M (1995) Distemper in wild carnivores—an epidemiologic, histological and immunocytochemical study. Vet Microbiol 44(2–4):193–199
Vicente J, Fierro Y, Martinez M, Gortazar C (2004a) Long-term epidemiology, effect on body condition and interspecific interactions of concomitant infection by nasopharyngeal bot fly larvae (Cephenemyia auribarbis and Pharyngomyia picta, Oestridae) in a population of Iberian red deer (Cervus elaphus hispanicus). Parasitology 129:349–361
Vicente J, Segalés J, Höfle U, Balasch M, Plana-Durán J, Domingo M, Gortazar C (2004b) Epidemiological study on porcine circovirus type 2 (PCV2) infection in the European wild boar (Sus scrofa). Vet Res 35:243–253
Vicente J, Ruiz-Fons F, Vidal D, Hofle U, Acevedo P, Villanua D, Fernandez-De-Mera IG, Martin MP, Gortazar C (2005) Serosurvey of Aujeszky’s disease virus infection in European wild boar in Spain. Vet Rec 156(13):408–412
Vicente J, Höfle U, Garrido JM, Fernández-De-Mera IG, Juste R, Barral M, Gortazar C (2006) Wild boar and red deer display high prevalences of tuberculosis-like lesions in Spain. Vet Res 37:107–119
Vicente J, Höfle U, Garrido JM, Fernández-De-Mera IG, Acevedo P, Juste R, Barral M, Gortazar C (2007) Risk factors associated with prevalence of tuberculosis-like lesions in wild boar and red deer in South Central Spain.Vet Res 38(3):451–464
Villanúa D, Acevedo P, Höfle U, Rodríguez O, Gortázar C (2006a) Changes in transmission stage excretion after pheasant release. J Helminthol 80:313–318
Villanúa D, Höfle U, Pérez-Rodríguez L, Gortázar C (2006b) Trichomonas gallinae in wintering common wood pigeons Columba palumbus in Spain. Ibis 148:641–648
Villanúa D, Casas F, Viñuela J, Gortazar C, García de la Morena E, Morales M (2007a) First occurrence of Eucoleus contortus in a little bustard Tetrax tetrax. A negative effect of red-legged partridge Alectoris rufa releases on steppe bird conservation? Ibis 149(2):405–406
Villanúa D, Pérez-Rodríguez L, Rodríguez O, Viñuela J, Gortázar C (2007b) How effective is pre-release nematode control in farm reared red-legged partridges (Alectoris rufa)? J Helminthol 31(1):101–103
Webster RG, Peiris M, Chen H, Guan Y (2006) H5N1 outbreaks and enzootic influenza. Emerg Infect Dis 12:3–8
Williams G, Wilkinson J (1998) Free-living wild boar in south-east England. Vet Rec 143(20):567
Williams D, Acevedo P, Gortázar C, Escudero MA, Labarta JL, Marco J, Villafuerte R (2007) Hunting for answers: rabbit (Oryctolagus cuniculus) population trends in northeastern Spain. E J Wildl Res 53(1):19–28. DOI 10.1007/s10344-006-0056-0
Wobeser GA (1994) Investigation and management of disease in wild animals. Plenum, New York, p 265
Wobeser GA (2002) Disease management strategies for wildlife. Rev Sci Tech Off Int Epizoot 21:159–178
Woodroffe FJ, Bourne DR, Cox CA, Donnelly G, Gettinby JP, McInerney A, Morrison WI (2005) Welfare of badgers (Meles meles) subjected to culling: patterns of trap-related injury. Anim Welf 14:11–17
Woolhouse ME (2002) Population biology of emerging and re-emerging pathogens. Trends Microbiol 10:3–7
Yamaguchi N, Macdonald DW (2001) Detection of Aleutian disease antibodies in feral American mink in southern England. Vet Rec 149:720
Acknowledgements
This review was presented as a plenary talk at the 7th Meeting of the European Section of the Wildlife Disease Association in Aosta, Italy, September 2006. This is a contribution to the agreement between IREC and MAPA/MMA on wildlife disease surveillance in Spain, to grant from MEC INIA (FAU2006-00017 and ACF2006-00001) and Plan Nacional (AGL2005-07401) on shared diseases (with FEDER) and to support from Junta de Comunidades de Castilla—La Mancha and from Grupo Santander. Quim Segalés and José Ardaiz are acknowledged for providing pictures to prepare Fig. 2.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by F. J. Kaup
Rights and permissions
About this article
Cite this article
Gortázar, C., Ferroglio, E., Höfle, U. et al. Diseases shared between wildlife and livestock: a European perspective. Eur J Wildl Res 53, 241–256 (2007). https://doi.org/10.1007/s10344-007-0098-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10344-007-0098-y