Abstract
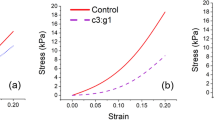
This study investigated the role of matrix metalloproteases and aggrecanases during dynamic compression-induced aggrecan catabolism in chondrocyte-seeded self-assembling peptide hydrogel. One- to two-week-old bovine chondrocytes were encapsulated into peptide hydrogel and cultured for 14 days prior to the application of an alternate day loading protocol. Dynamic compression-induced aggrecan catabolism was explored by evaluating GAG loss to the culture medium, zymography for matrix metalloproteases (MMPs), gene expression of MMPs and ADAMTS proteases, and Western blot analysis for aggrecan fragments. The application of loading over 4 days increased GAG loss to the medium three- to four-fold relative to free-swelling controls. Zymogram analysis detected increased concentrations of latent MMP-9 and MMP-3 in the culture medium relative to free-swelling culture. Real-time PCR showed expression levels of MMPs and ADAMTS proteases in loaded samples that ranged from 2.5- to 95-fold higher than free-swelling culture. Aggrecan fragment analysis did not detect small (50–80 kDa) molecular weight fragments in free-swelling culture; however, dynamic compression samples contained 60–80 kDa fragments that were detected by both anti-G1 and NITEGE probes, demonstrating ADAMTS but not MMP degradation. These data suggest that partially mature cartilage tissue engineering constructs may be susceptible to catabolic degradation.




Similar content being viewed by others
References
Abbaszade I, Liu RQ, Yang F, et al (1999) Cloning and characterization of ADAMTS11, an aggrecanase from the ADAMTS family. J Biol Chem 274:23443-23450. doi:10.1074/jbc.274.33.23443
Arner EC, Hughes CE, Decicco CP, et al (1998) Cytokine-induced cartilage proteoglycan degradation is mediated by aggrecanase. Osteoarthritis Cartilage 6:214-228. doi:10.1053/joca.1998.0114
Bau B, Gebhard PM, Haag J, et al (2002) Relative messenger RNA expression profiling of collagenases and aggrecanases in human articular chondrocytes in vivo and in vitro. Arthritis Rheum 46:2648-2657. doi:10.1002/art.10531
Billinghurst RC, Dahlberg L, Ionescu M, et al (1997) Enhanced cleavage of type II collagen by collagenases in osteoarthritic articular cartilage. J Clin Invest 99:1534-1545. doi:10.1172/JCI119316
Blain EJ, Gilbert SJ, Wardale RJ, et al (2001) Up-regulation of matrix metalloproteinase expression and activation following cyclical compressive loading of articular cartilage in vitro. Arch Biochem Biophys 396:49-55. doi:10.1006/abbi.2001.2575
Buschmann MD, Gluzband YA, Grodzinsky AJ, et al (1995) Mechanical compression modulates matrix biosynthesis in chondrocyte/agarose culture. J Cell Sci 108(Pt 4):1497-1508
Butler DL, Goldstein SA, Guilak F (2000) Functional tissue engineering: the role of biomechanics. J Biomech Eng 122:570-575. doi:10.1115/1.1318906
Candrian C, Vonwil D, Barbero A, et al (2008) Engineered cartilage generated by nasal chondrocytes is responsive to physical forces resembling joint loading. Arthritis Rheum 58:197-208. doi:10.1002/art.23155
Caterson B, Flannery CR, Hughes CE, et al (2000) Mechanisms involved in cartilage proteoglycan catabolism. Matrix Biol 19:333-344. doi:10.1016/S0945-053X(00)00078-0
Chockalingam PS, Zeng W, Morris EA, et al (2004) Release of hyaluronan and hyaladherins (aggrecan G1 domain and link proteins) from articular cartilage exposed to ADAMTS-4 (aggrecanase 1) or ADAMTS-5 (aggrecanase 2). Arthritis Rheum 50:2839-2848. doi:10.1002/art.20496
Clark JM, Norman A, Notzli H (1997) Postnatal development of the collagen matrix in rabbit tibial plateau articular cartilage. J Anat 191 (Pt 2):215-221. doi:10.1046/j.1469-7580.1997.19120215.x
Davisson T, Kunig S, Chen A, et al (2002) Static and dynamic compression modulate matrix metabolism in tissue engineered cartilage. J Orthop Res 20:842-848. doi:10.1016/S0736-0266(01)00160-7
De Croos JN, Dhaliwal SS, Grynpas MD, et al (2006) Cyclic compressive mechanical stimulation induces sequential catabolic and anabolic gene changes in chondrocytes resulting in increased extracellular matrix accumulation. Matrix Biol 25:323-331. doi:10.1016/j.matbio.2006.03.005
Demarteau O, Wendt D, Braccini A, et al (2003) Dynamic compression of cartilage constructs engineered from expanded human articular chondrocytes. Biochem Biophys Res Commun 310:580-588. doi:10.1016/j.bbrc.2003.09.099
DiMicco MA, Patwari P, Siparsky PN, et al (2004) Mechanisms and kinetics of glycosaminoglycan release following in vitro cartilage injury. Arthritis Rheum 50:840-848. doi:10.1002/art.20101
D’Lima DD, Hashimoto S, Chen PC, et al (2001) Human chondrocyte apoptosis in response to mechanical injury. Osteoarthritis Cartilage 9:712-719. doi:10.1053/joca.2001.0468
Fitzgerald JB, Jin M, Dean D, et al (2004) Mechanical compression of cartilage explants induces multiple time-dependent gene expression patterns and involves intracellular calcium and cyclic AMP. J Biol Chem 279:19502-19511. doi:10.1074/jbc.M400437200
Fitzgerald, J. B., M. Jin, and A. J. Grodzinsky. Shear and compression differentially regulate clusters of functionally-related temporal transcription patterns in cartilage tissue. J. Biol. Chem. 281(34):24095–24103, 2006.
Flannery CR, Lark MW, Sandy JD (1992) Identification of a stromelysin cleavage site within the interglobular domain of human aggrecan. Evidence for proteolysis at this site in vivo in human articular cartilage. J Biol Chem 267:1008-1014
Flannery CR, Little CB, Hughes CE, et al (1999) Expression of ADAMTS homologues in articular cartilage. Biochem Biophys Res Commun 260:318-322. doi:10.1006/bbrc.1999.0909
Frank EH, Jin M, Loening AM, et al (2000) A versatile shear and compression apparatus for mechanical stimulation of tissue culture explants. J Biomech 33:1523-1527. doi:10.1016/S0021-9290(00)00100-7
Freemont AJ, Hampson V, Tilman R, et al (1997) Gene expression of matrix metalloproteinases 1, 3, and 9 by chondrocytes in osteoarthritic human knee articular cartilage is zone and grade specific. Ann Rheum Dis 56:542-549. doi:10.1136/ard.56.9.542
Gilbert SJ, Wotton PR, Tarlton JF, et al (1997) Increased expression of promatrix metalloproteinase-9 and neutrophil elastase in canine dilated cardiomyopathy. Cardiovasc Res 34:377-383. doi:10.1016/S0008-6363(97)00011-4
Glasson SS, Askew R, Sheppard B, et al (2004) Characterization of and osteoarthritis susceptibility in ADAMTS-4-knockout mice. Arthritis Rheum 50:2547-2558. doi:10.1002/art.20558
Grodzinsky AJ, Levenston ME, Jin M, et al (2000) Cartilage tissue remodeling in response to mechanical forces. Annu Rev Biomed Eng 2:691-713. doi:10.1146/annurev.bioeng.2.1.691
Hascall VC, Handley CJ, McQuillan DJ, et al (1983) The effect of serum on biosynthesis of proteoglycans by bovine articular cartilage in culture. Arch Biochem Biophys 224:206-223. doi:10.1016/0003-9861(83)90205-9
Hunter CJ, Mouw JK, Levenston ME (2004) Dynamic compression of chondrocyte-seeded fibrin gels: effects on matrix accumulation and mechanical stiffness. Osteoarthritis Cartilage 12:117-130. doi:10.1016/j.joca.2003.08.009
Hunziker EB (2002) Articular cartilage repair: basic science and clinical progress. A review of the current status and prospects. Osteoarthritis Cartilage 10:432-463. doi:10.1053/joca.2002.0801
Karsdal MA, Madsen SH, Christiansen C, et al (2008) Cartilage degradation is fully reversible in the presence of aggrecanase but not matrix metalloproteinase activity. Arthritis Res Ther 10:R63. doi:10.1186/ar2434
Kisiday J, Jin M, Kurz B, et al (2002) Self-assembling peptide hydrogel fosters chondrocyte extracellular matrix production and cell division: implications for cartilage tissue repair. Proc Natl Acad Sci U S A 99:9996-10001. doi:10.1073/pnas.142309999
Kisiday JD, Jin M, DiMicco MA, et al (2004) Effects of dynamic compressive loading on chondrocyte biosynthesis in self-assembling peptide scaffolds. J Biomech 37:595-604. doi:10.1016/j.jbiomech.2003.10.005
Kisiday JD, Kurz B, DiMicco MA, et al (2005) Evaluation of medium supplemented with insulin-transferrin-selenium for culture of primary bovine calf chondrocytes in three-dimensional hydrogel scaffolds. Tissue Eng 11:141-151. doi:10.1089/ten.2005.11.141
Klein, T. J., M. Chaudhry, W. C. Bae, et al. Depth-dependent biomechanical and biochemical properties of fetal, newborn, and tissue-engineered articular cartilage. J. Biomech. 40(1):182–190, 2005.
Kurz B, Jin M, Patwari P, et al (2001) Biosynthetic response and mechanical properties of articular cartilage after injurious compression. J Orthop Res 19:1140-1146. doi:10.1016/S0736-0266(01)00033-X
Lee CR, Grodzinsky AJ, Spector M (2003) Biosynthetic response of passaged chondrocytes in a type II collagen scaffold to mechanical compression. J Biomed Mater Res A 64:560-569. doi:10.1002/jbm.a.10443
Lee DA, Bader DL (1997) Compressive strains at physiological frequencies influence the metabolism of chondrocytes seeded in agarose. J Orthop Res 15:181-188. doi:10.1002/jor.1100150205
Lee JH, Fitzgerald JB, Dimicco MA, et al (2005) Mechanical injury of cartilage explants causes specific time-dependent changes in chondrocyte gene expression. Arthritis Rheum 52:2386-2395. doi:10.1002/art.21215
Little CB, Flannery CR, Hughes CE, et al (1999) Aggrecanase versus matrix metalloproteinases in the catabolism of the interglobular domain of aggrecan in vitro. Biochem J 344(Pt 1):61-68. doi:10.1042/0264-6021:3440061
Little CB, Flannery CR, Hughes CE, et al (2005) Cytokine induced metalloproteinase expression and activity does not correlate with focal susceptibility of articular cartilage to degeneration. Osteoarthritis Cartilage 13:162-170. doi:10.1016/j.joca.2004.10.014
Malfait AM, Liu RQ, Ijiri K, et al (2002) Inhibition of ADAM-TS4 and ADAM-TS5 prevents aggrecan degradation in osteoarthritic cartilage. J Biol Chem 277:22201-22208. doi:10.1074/jbc.M200431200
Mauck RL, Soltz MA, Wang CC, et al (2000) Functional tissue engineering of articular cartilage through dynamic loading of chondrocyte-seeded agarose gels. J Biomech Eng 122:252-260. doi:10.1115/1.429656
Milner JM, Rowan AD, Cawston TE, et al (2006) Metalloproteinase and inhibitor expression profiling of resorbing cartilage reveals pro-collagenase activation as a critical step for collagenolysis. Arthritis Res Ther 8:R142. doi:10.1186/ar2034
Patwari P, Gao G, Lee JH, et al (2005) Analysis of ADAMTS4 and MT4-MMP indicates that both are involved in aggrecanolysis in interleukin-1-treated bovine cartilage. Osteoarthritis Cartilage 13:269-277. doi:10.1016/j.joca.2004.10.023
Powell AJ, Little CB, Hughes CE (2007) Low molecular weight isoforms of the aggrecanases are responsible for the cytokine-induced proteolysis of aggrecan in a porcine chondrocyte culture system. Arthritis Rheum 56:3010-3019. doi:10.1002/art.22818
Sah RL, Kim YJ, Doong JY, et al (1989) Biosynthetic response of cartilage explants to dynamic compression. J Orthop Res 7:619-636. doi:10.1002/jor.1100070502
Sandy JD (2006) A contentious issue finds some clarity: on the independent and complementary roles of aggrecanase activity and MMP activity in human joint aggrecanolysis. Osteoarthritis Cartilage 14:95-100. doi:10.1016/j.joca.2005.09.004
Sandy JD, Neame PJ, Boynton RE, et al (1991) Catabolism of aggrecan in cartilage explants. Identification of a major cleavage site within the interglobular domain. J Biol Chem 266:8683-8685
Sandy JD, Verscharen C (2001) Analysis of aggrecan in human knee cartilage and synovial fluid indicates that aggrecanase (ADAMTS) activity is responsible for the catabolic turnover and loss of whole aggrecan whereas other protease activity is required for C-terminal processing in vivo. Biochem J 358:615-626. doi:10.1042/0264-6021:3580615
Tortorella MD, Burn TC, Pratta MA, et al (1999) Purification and cloning of aggrecanase-1: a member of the ADAMTS family of proteins. Science 284:1664-1666. doi:10.1126/science.284.5420.1664
Tortorella MD, Malfait AM, Deccico C, et al (2001) The role of ADAM-TS4 (aggrecanase-1) and ADAM-TS5 (aggrecanase-2) in a model of cartilage degradation. Osteoarthritis Cartilage 9:539-552. doi:10.1053/joca.2001.0427
Acknowledgment
NIH-BRP grant EB003805 (MIT). NIH AR33236 (MIT).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kisiday, J.D., Lee, J.H., Siparsky, P.N. et al. Catabolic Responses of Chondrocyte-Seeded Peptide Hydrogel to Dynamic Compression. Ann Biomed Eng 37, 1368–1375 (2009). https://doi.org/10.1007/s10439-009-9699-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-009-9699-9