Abstract
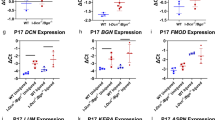
During post-natal development, tendons undergo a well orchestrated process whereby extensive structural and compositional changes occur in synchrony to produce a normal tissue. Conversely, during the repair response to injury, structural and compositional changes occur, but in this case, a mechanically inferior tendon is produced. As a result, the process of development has been postulated as a potential paradigm through which improved adult tissue healing may occur. In this study we measured the mechanical, compositional, and structural properties in the post-natal mouse Achilles tendon at 4, 7, 10, 14, 21, and 28 days old. Throughout post-natal development, the mechanical properties, collagen content, fibril diameter mean, and fibril diameter standard deviation increased. Biglycan expression decreased and decorin expression and fiber organization were unchanged. This study provides a new mouse model that can be used to quantitatively examine mechanical development, as well as compositional and structural changes and biological mechanisms, during post-natal tendon development. This model is advantageous due to the large number of genetically modified mice and commercially available assays that are not available in other animal models. A mouse model therefore allows future mechanistic studies to build on this work.







Similar content being viewed by others
References
Ansorge, H. L., X. Meng, G. Zhang, G. Veit, M. Sun, J. F. Klement, D. P. Beason, L. J. Soslowsky, M. Koch, and D. E. Birk. Type XIV collagen regulates fibrillogenesis: premature collagen fibril growth and tissue dysfunction in null mice. J. Biol. Chem. 284:8427–8438, 2009.
Beredjiklian, P. K. Biologic aspects of flexor tendon laceration and repair. J. Bone Joint Surg. Am. 85-A:539–550, 2003.
Birk, D. E., R. A. Hahn, C. Y. Linsenmayer, and E. I. Zycband. Characterization of collagen fibril segments from chicken embryo cornea, dermis and tendon. Matrix Biol. 15:111–118, 1996.
Birk, D. E., and R. Mayne. Localization of collagen types I, III and V during tendon development. Changes in collagen types I and III are correlated with changes in fibril diameter. Eur. J. Cell Biol. 72:352–361, 1997.
Birk, D. E., E. I. Zycband, D. A. Winkelmann, and R. L. Trelstad. Collagen fibrillogenesis in situ: fibril segments are intermediates in matrix assembly. Proc. Natl Acad. Sci. USA 86:4549–4553, 1989.
Bland, Y. S., and D. E. Ashhurst. Fetal and postnatal development of the patella, patellar tendon and suprapatella in the rabbit; changes in the distribution of the fibrillar collagens. J. Anat. 190(Pt 3):327–342, 1997.
Booth, F. W., and C. M. Tipton. Ligamentous strength measurements in pre-pubescent and pubescent rats. Growth 34:177–185, 1970.
Boykiw, R., P. Sciore, C. Reno, L. Marchuk, C. B. Frank, and D. A. Hart. Altered levels of extracellular matrix molecule mRNA in healing rabbit ligaments. Matrix Biol. 17:371–378, 1998.
Danielson, K. G., H. Baribault, D. F. Holmes, H. Graham, K. E. Kadler, and R. V. Iozzo. Targeted disruption of decorin leads to abnormal collagen fibril morphology and skin fragility. J. Cell Biol. 136:729–743, 1997.
Derwin, K. A., and L. J. Soslowsky. A quantitative investigation of structure–function relationships in a tendon fascicle model. J. Biomech. Eng. 121:598–604, 1999.
Derwin, K. A., L. J. Soslowsky, W. D. Green, and S. H. Elder. A new optical system for the determination of deformations and strains: calibration characteristics and experimental results. J. Biomech. 27:1277–1285, 1994.
Ehrlich, H. P., P. A. Lambert, G. C. Saggers, R. L. Myers, and R. M. Hauck. Dynamic changes appearing in collagen fibers during intrinsic tendon repair. Ann. Plast. Surg. 54:201–206, 2005.
Ezura, Y., S. Chakravarti, A. Oldberg, I. Chervoneva, and D. E. Birk. Differential expression of lumican and fibromodulin regulate collagen fibrillogenesis in developing mouse tendons. J. Cell Biol. 151:779–788, 2000.
Festing, M. F. Design and statistical methods in studies using animal models of development. ILAR J. 47:5–14, 2006.
Franchi, M., M. Fini, M. Quaranta, V. De Pasquale, M. Raspanti, G. Giavaresi, V. Ottani, and A. Ruggeri. Crimp morphology in relaxed and stretched rat Achilles tendon. J. Anat. 210:1–7, 2007.
Gimbel, J. A., J. P. Van Kleunen, S. Mehta, S. M. Perry, G. R. Williams, and L. J. Soslowsky. Supraspinatus tendon organizational and mechanical properties in a chronic rotator cuff tear animal model. J. Biomech. 37:739–749, 2004.
Lin, T. W., L. Cardenas, and L. J. Soslowsky. Tendon properties in interleukin-4 and interleukin-6 knockout mice. J. Biomech. 38:99–105, 2005.
McBride, D. J., R. L. Trelstad, and F. H. Silver. Structural and mechanical assessment of developing chick tendon. Int. J. Biol. Macromol. 10:194–200, 1988.
Mikic, B., E. Amadei, K. Rossmeier, and L. Bierwert. Sex matters in the establishment of murine tendon composition and material properties during growth. J. Orthop. Res. 28:631–638, 2010.
Moore, M. J., and A. De Beaux. A quantitative ultrastructural study of rat tendon from birth to maturity. J. Anat. 153:163–169, 1987.
Neuman, R. E., and M. A. Logan. The determination of hydroxyproline. J. Biol. Chem. 184:299–306, 1950.
Oryan, A., and A. H. Shoushtari. Histology and ultrastructure of the developing superficial digital flexor tendon in rabbits. Anat. Histol. Embryol. 37:134–140, 2008.
Parry, D. A., G. R. Barnes, and A. S. Craig. A comparison of the size distribution of collagen fibrils in connective tissues as a function of age and a possible relation between fibril size distribution and mechanical properties. Proc. R. Soc. Lond. B Biol. Sci. 203:305–321, 1978.
Parry, D. A., A. S. Craig, and G. R. Barnes. Tendon and ligament from the horse: an ultrastructural study of collagen fibrils and elastic fibres as a function of age. Proc. R. Soc. Lond. B Biol. Sci. 203:293–303, 1978.
Peltz, C. D., S. M. Perry, C. L. Getz, and L. J. Soslowsky. Mechanical properties of the long-head of the biceps tendon are altered in the presence of rotator cuff tears in a rat model. J. Orthop. Res. 27:416–420, 2009.
Rigozzi, S., R. Muller, and J. G. Snedeker. Collagen fibril morphology and mechanical properties of the Achilles tendon in two inbred mouse strains. J. Anat. 216:724–731, 2010.
Thomopoulos, S., G. Hattersley, V. Rosen, M. Mertens, L. Galatz, G. R. Williams, and L. J. Soslowsky. The localized expression of extracellular matrix components in healing tendon insertion sites: an in situ hybridization study. J. Orthop. Res. 20:454–463, 2002.
Thomopoulos, S., G. R. Williams, J. A. Gimbel, M. Favata, and L. J. Soslowsky. Variation of biomechanical, structural, and compositional properties along the tendon to bone insertion site. J. Orthop. Res. 21:413–419, 2003.
Webster, T. Putting a strain on workers’ health. Compens. Work. Cond. Spring:29–31, 1999. http://www.bls.gov/opub/cwc/archive/spring1999brief2.pdf.
Woo, S. L., R. E. Debski, J. Zeminski, S. D. Abramowitch, S. S. Saw, and J. A. Fenwick. Injury and repair of ligaments and tendons. Annu. Rev. Biomed. Eng. 2:83–118, 2000.
Woo, S. L., R. H. Gelberman, N. G. Cobb, D. Amiel, K. Lothringer, and W. H. Akeson. The importance of controlled passive mobilization on flexor tendon healing. A biomechanical study. Acta Orthop. Scand. 52:615–622, 1981.
Woo, S. L., C. A. Orlando, M. A. Gomez, C. B. Frank, and W. H. Akeson. Tensile properties of the medial collateral ligament as a function of age. J. Orthop. Res. 4:133–141, 1986.
Zhang, G., S. Chen, S. Goldoni, B. W. Calder, H. C. Simpson, R. T. Owens, D. J. McQuillan, M. F. Young, R. V. Iozzo, and D. E. Birk. Genetic evidence for the coordinated regulation of collagen fibrillogenesis in the cornea by decorin and biglycan. J. Biol. Chem. 284:8888–8897, 2009.
Zhang, G., Y. Ezura, I. Chervoneva, P. S. Robinson, D. P. Beason, E. T. Carine, L. J. Soslowsky, R. V. Iozzo, and D. E. Birk. Decorin regulates assembly of collagen fibrils and acquisition of biomechanical properties during tendon development. J. Cell Biochem. 98:1436–1449, 2006.
Zhang, G., B. B. Young, Y. Ezura, M. Favata, L. J. Soslowsky, S. Chakravarti, and D. E. Birk. Development of tendon structure and function: regulation of collagen fibrillogenesis. J. Musculoskelet. Neuronal Interact. 5:5–21, 2005.
Acknowledgments
This work was supported, in whole or in part, by National Institutes of Health Grant AR050950 from NIAMS, supporting the Penn Center for Musculoskeletal Disorders and by National Institutes of Health Grant AR44745. The authors would like to thank David Beason for his expertise in helping develop the mechanical testing fixtures.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Eric M. Darling oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Ansorge, H.L., Adams, S., Birk, D.E. et al. Mechanical, Compositional, and Structural Properties of the Post-natal Mouse Achilles Tendon. Ann Biomed Eng 39, 1904–1913 (2011). https://doi.org/10.1007/s10439-011-0299-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-011-0299-0