Abstract
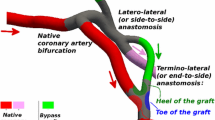
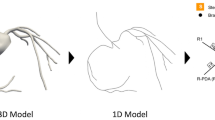
We present a computational framework for multiscale modeling and simulation of blood flow in coronary artery bypass graft (CABG) patients. Using this framework, only CT and non-invasive clinical measurements are required without the need to assume pressure and/or flow waveforms in the coronaries and we can capture global circulatory dynamics. We demonstrate this methodology in a case study of a patient with multiple CABGs. A patient-specific model of the blood vessels is constructed from CT image data to include the aorta, aortic branch vessels (brachiocephalic artery and carotids), the coronary arteries and multiple bypass grafts. The rest of the circulatory system is modeled using a lumped parameter network (LPN) 0 dimensional (0D) system comprised of resistances, capacitors (compliance), inductors (inertance), elastance and diodes (valves) that are tuned to match patient-specific clinical data. A finite element solver is used to compute blood flow and pressure in the 3D (3 dimensional) model, and this solver is implicitly coupled to the 0D LPN code at all inlets and outlets. By systematically parameterizing the graft geometry, we evaluate the influence of graft shape on the local hemodynamics, and global circulatory dynamics. Virtual manipulation of graft geometry is automated using Bezier splines and control points along the pathlines. Using this framework, we quantify wall shear stress, wall shear stress gradients and oscillatory shear index for different surgical geometries. We also compare pressures, flow rates and ventricular pressure–volume loops pre- and post-bypass graft surgery. We observe that PV loops do not change significantly after CABG but that both coronary perfusion and local hemodynamic parameters near the anastomosis region change substantially. Implications for future patient-specific optimization of CABG are discussed.













Similar content being viewed by others
References
Agoshkov, V., A. Quarteroni, and G. Rozza. A mathematical approach in the design of arterial bypass anastomosis using unsteady stokes equations. J. Sci. Comput. 38:139–161, 2006.
Bassiouny, H., S. White, S. Glagov, E. Choi, D. P. Giddens, and C. K. Zarins. Anastomotic intimal hyperplasia: mechanical injury or flow induced. J. Vasc. Surg. 10:326–337, 1989.
Bazilevs, Y., M.-C. Hsu, D. Besnon, S. Sankaran, and A. Marsden. Computational fluid-structure interaction: Methods and application to a total cavopulmonary connection. Comput. Mech. 45(1):77–99, 2009.
Bogren, H., R. Klipstein, D. Firmin, R. Mohiaddin, S. Underwood, R. Rees, and D. Longmore. Quantitation of antegrade and retrograde blood-flow in the human aorta by magnetic-resonance velocity mapping. Am. Heart J. 117(6):1214–1222, 1989.
Bryan, A., and G. D. Angelini. The biology of saphenous vein graft occlusion: etiology and strategies for prevention. Curr. Opin. Cardiol. 9:641–649, 1994.
Burattini, R., P. Sipkema, G. Vanhuis, and N. Westerhof. Identification of canine coronary resistance and intramyocardial compliance on the basis of the waterfall model. Ann. Biomed. Eng. 13(5):385–404, 1985.
DePaola, N., M. A. Gimbrone, Jr., P. F. Davies, and C. F. Dewey, Jr. Vascular endothelium responds to fluid shear stress gradients. Arter. Thromb. Vasc. Biol. 12:1254–1257, 1992
Dur, O., S. Coskun, K. Coskun, D. Frakes, L. Kara, and K. Pekkan. Computer-aided patient-specific coronary artery graft design improvements using CFD coupled shape optimizer. Cardiovasc. Eng. Technol. 2(1):35–47, 2011.
Esmaily Moghadam, M., Y. Bazilevs, T.-Y. Hsia, I. Vignon-Clementel, A. Marsden, and Modeling of Congenital Hearts Alliance (MOCHA). A comparison of outlet boundary treatments for prevention of backflow divergence with relevance to blood flow simulations. Comput. Mech. 48(3):277–291, 2011.
Esmaily Moghadam, M., I. Vignon-Clementel, R. Figliola, and A. Marsden. A modular numerical method for implicit 0D/3D coupling in cardiovascular finite element simulations. J. Comput. Phys., 2011, in review.
Giddens, D., C. K. Zarins, and S. Glagov. The role of fluid mechanics in localization and detection of atherosclerosis. J. Biomech. Eng. 115:588–594, 1993.
Giordana, S., S. Sherwin, J. Pelro, D. Doorly, J. Crane, K. Lee, N. Cheshire, and C. Caro. Local and global geometric influence on steady flow in distal anastomoses of peripheral bypass grafts. J. Biomech. Eng. 127:1087–1098, 2005.
Haruguchi, H., and S. Teraoka. Intimal hyperplasia and hemodynamic factors in arterial bypass and arteriovenous grafts: a review. J. Artifi. Organs 6:227–235, 2003.
Holzapfel, G. A., T. Gasser, and T. Ogden. A new constitutive framework for arterial wall mechanics and a comparative study of material models. J. Elast. 61:1–48, 2000.
Inzoli, F., F. Migliavacca, and G. Pennati. Numerical analysis of steady flow in aorto-coronary bypass 3D model. J. Biomech. Eng. 118:172–179, 1996.
Jansen, K. E., C. H. Whiting, and G. M. Hulbert. A generalized-alpha method for integrating the filtered Navier Stokes equations with a stabilized finite element method. Comput. Methods Appl. Mech. Eng. 190(3–4):305–319, 2000.
Johnson, K., P. Sharma, and J. Oshinski. Coronary artery flow measurement using navigator echo gated phase contrast magnetic resonance velocity mapping at 3.0 t. J. Biomech. 41:595–602, 2008.
Katia, L., R. Balossino, F. Migliavacca, G. Pennati, E. Bove, M. de Leval, and G. Dubini. Multiscale modeling of the cardiovascular system: application to the study of pulmonary and coronary perfusions in the univentricular circulation. J. Biomech. 28:1129–1141, 2004.
Keynton, R., M. Evancho, R. Sims, N. Rodway, A. Gobin, and S. Rittgers. Intimal hyperplasia and wall shear in arterial bypass graft distal anastomosis: an in vivo model study. J. Biomech. Eng. 123:464–473, 2001.
Kim, H., I. E. Vignon-Clementel, J. Coogan, C. Figueroa, K. Jansen, and C. Taylor. On coupling a lumped parameter heart model and a three-dimensional finite element aorta model. Ann. Biomed. Eng. 37(11):2153–2169, 2009.
Kim, H., I. Vignon-Clementel, A. Figueroa, J. Ladisa, K. Jansen, J. Feinstein, and C. Taylor. Patient-specific modeling of blood flow and pressure in human coronary arteries. Ann. Biomed. Eng. 38(10):3195–3209, 2010.
Kohler, T., T. R. Kirkman, L. W. Kraiss, B. K. Zierler, and A. W. Clowes. Increased blood flow inhibits neointimal hyperplasia in endothelialized vascular grafts. Circ. Res. 69:1557–1565, 1991.
Ku, D. Blood flow in arteries. Ann. Rev. Fluid Mech. 29:399–434, 1997.
Lagan, K., G. Dubini, F. Migliavacca, R. Pietrabissa, G. Pennati, A. Veneziani, and A. Quarteroni. Multiscale modelling as a tool to prescribe realistic boundary conditions for the study of surgical procedures. Biorheology 39:359–364, 2002.
Lei, M., J. Archie, and C. Kleinstreur. Computational design of a bypass graft that minimizes wall shear stress gradients in the region of the distal anastomosis. J. Vasc. Surg. 25(4):637–646, 1997.
Liu, M., G. S. Roubin, and S. King. Restenosis after coronary angioplasty: potential biologic determinants and role of intimal hyperplasia. Circulation 79:1374–1387, 1989.
Loth, F., P. F. Fischer, and H. S. Bassiouny. Blood flow in end-to-side anastomosis. Ann. Rev. Fluid Mech. 40:367–393, 2008.
Loth, F., S. A. Jones, C. K. Zarins, D. P. Giddens, R. F. Nassar, S. Glagov, and H. S. Bassiouny. Relative contribution of wall shear stress and injury in experimental intimal thickening at PTFE end-to-side arterial anastomoses. J. Biomech. Eng. 124:44–51, 2002.
Malek, A. M., S. L. Alper, and S. Izumo. Hemodynamic shear stress and its role in atherosclerosis. J. Am. Med. Assoc. 282(21):2035–2042, 1999.
Manthaa, A., C. Karmonikc, G. Benndorfb, C. Strotherc, and R. Metcalfea. Hemodynamics in a cerebral artery before and after the formation of an aneurysm. Am. J. Neuroradiol. 27:1113–1118, 2006.
Marsden, A., J. Feinstein, and C. Taylor. A computational framework for derivative-free optimization of cardiovascular geometries. Comput. Methods Appl. Mech. Eng. 197(21–24):1890–1905, 2008.
Mehta, D., M. B. Izzat, A. J. Bryan, and G. D. Angelini. Towards the prevention of vein graft failure. Int. J. Cardiol. 62(1):S55–S63, 1997.
Migliavacca, F., and G. Dubini. Computational modeling of vascular anastomoses. Biomech. Model. Mechanobiol. 3:235–250, 2005.
Moore, J., D. A. Steinman, S. Prakash, K. W. Johnson, and C. R. Ethier. A numerical study of blood flow patterns in anatomically realistic and simplified end-to-side anastomoses. J. Biomech. Eng. 121:265–272, 1999.
Neal, M., and J. Bassingthwaighte. Subject-specific model estimation of cardiac output and blood volume during hemorrhage. Cardiovasc. Eng. 7(3):97–120, 2007.
Nikkari, S., and A. W. Clowes. Restenosis after vascular reconstruction. Ann. Med. 26:95–100, 1994.
Ojha, M. Spatial and temporal variations of wall shear stress within an end-to-side arterial anastomosis model. J. Biomech. 26(12):1377–1388, 1993.
Ojha, M. Wall shear stress temporal gradient and anastomotic intimal hyperplasia. Circ. Res. 74:1227–1231, 1994.
Rittgers, S., P. Karayannacos, J. Guy, R. Nerem, G. Shaw, J. Hostetler, and J. Vasko. Velocity distribution and intimal proliferation in autologous vein grafts in dogs. Circ. Res. 42(6):792–801, 1978.
Samady, H., P. Eshtehardi, M. McDaniel, J. Suo, S. Dhawan, C. Maynard, L. Timmins, A. Quyyumi, and D. Giddens. Coronary artery wall shear stress is associated with progression and transformation of atherosclerotic plaque and arterial remodeling in patients with coronary artery disease. Circulation 124:779–788, 2011.
Sankaran, S., and A. Marsden. The impact of uncertainty on shape optimization of idealized bypass graft models in unsteady flow. Phys. Fluids 22:121902, 2010.
Sankaran, S., and A. Marsden. A stochastic collocation method for uncertainty quantification and propagation in cardiovascular simulations. J. Biomech. Eng. 133:031001, 2011.
Sankaranarayanan, M., D. Ghista, C. Poh, T. Seng, and G. Kassab. Analysis of blood flow in an out-of-plane CABG model. Am. J. Physiol. Heart Circ. Physiol. 291:H283–H295, 2006
Senzaki, H., C. H. Chen, and D. A. Kass. Single beat estimation of end-systolic pressure-volume relation in humans: a new method with the potential for noninvasive application. Circulation 94(10):2497–2506, 1996.
Shimogonya, Y., T. Ishikawa, Y. Imai, N. Matsuki, and T. Yamaguchi. Can temporal fluctuation in spatial wall shear stress gradient initiate a cerebral aneurysm? A proposed novel hemodynamic index, the gradient oscillatory number (GON). J. Biomech. 42(4):550–554, 2009.
Siogkas, P. K., A. I. Sakellarios, T. P. Exarchos, K. Stefanou, D. I. Fotiadis, K. Naka, L. Michalis, N. Filipovic, and O. Parodi. Blood flow in arterial segments: rigid vs. deformable walls simulations. J. Serbian Soc. Comput. Mech. 5(1):69–77, 2011.
Staalsen, N., M. Ulrich, J. Winther, E. Pedersen, T. How, and H. Nygaard. The anastomosis angle does change the flow fields at vascular end-to-side anastomoses in vivo. J. Vasc. Surg. 21:460–471, 1995.
Taylor, C., M. Draney, J. Ku, D. Parker, B. Steele, K. Wang, and C. Zarins. Predictive medicine: computational techniques in therapeutic decision-making. Comput. Aided Surg. 4(5):231–247, 1999.
Taylor, C. A., T. J. R. Hughes, and C. K. Zarins. Finite element modeling of blood flow in arteries. Comput. Methods Appl. Mech. Eng. 158(1–2):155–196, 1997.
Walker, J., M. B. Ratcliffe, P. Zhang, A. W. Wallace, B. Fata, E. W. Hsu, D. Saloner, and J. M. Guccione. MRI-based finite-element analysis of left ventricular aneurysm. Am. J. Physiol. 289:H692–H700, 2005.
Wilson, N., K. Wang, R. Dutton, and C. A. Taylor. A software framework for creating patient specific geometric models from medical imaging data for simulation based medical planning of vascular surgery. Lect. Notes Comput. Sci. 2208:449–456, 2001.
Yang, W., I. Vignon-Clementel, G. Troianowski, V. Mohan Reddy, J. Feinstein, and A. Marsden. Hepatic blood flow distribution and performance in conventional and novel Y-graft Fontan geometries: a case series computational fluid dynamics study. J. Thorac. Cardiovasc. Surg. 143(5):1086–1097, 2012.
Zamir, M., P. Sinclair, and T. H. Wonnacott. Relation between diameter and flow in major branches of the arch of the aorta. J. Biomech. 25:1303–1310, 1992.
Zarins, C., M. A. Zatina, D. P. Giddens, D. N. Ku, and S. Glagov. Shear stress regulation of artery lumen diameter in experimental atherogenesis. J. Vasc. Surg. 5:413–420, 1987.
Acknowledgments
The authors acknowledge funding from an American Heart Association postdoctoral fellowship, the Leducq Foundation, a Burroughs Wellcome Fund Career Award at the Scientific Interface, and NIH grant RHL102596A for funding this work. We thank Hyun Jin Kim for expertise on coronary modeling, and Francesco Migliavacca for expertise on multiscale modeling. Dr. Guccione acknowledges funding from the National Institute of Health, grant numbers R01HL077921 and R01HL086400.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor John H. Linehan oversaw the review of this article.
Appendix
Appendix
Here, we provide details on computing the WSS gradients used in this study. For each element e, let the normal be denoted by n e and the mean shear stress be denoted by \(\bar{\tau}^e = \,\parallel \bar{\tau} \parallel{s^{e}_1}. \) The direction s e2 is computed as \(s^{e}_2=\frac{s^{e}_{1} \times n^{e}}{\parallel s^{e}_{1} \times n^{e} \parallel }. \) The components of elemental shear stress in these two directions are computed by taking a dot product with the directions. We can compute the gradients as follows:
The gradient of the shear stresses are evaluated at each tetrahedral element as:
The corresponding MWSSG is represented as \(\overline{\tau^{G,e}}. \) We then derive the nodal values as
Discretizing this equation and choosing the test functions as shape functions, we have
where A denotes the assembly matrix. Integrating this equation, we observe that:
This equation is found from the least squares method (minimizing the difference between τG and τG,e). For the purpose of visualization, we approximate the LHS system to choose nodal values that are weighted averages of the elemental solutions.
Rights and permissions
About this article
Cite this article
Sankaran, S., Esmaily Moghadam, M., Kahn, A.M. et al. Patient-Specific Multiscale Modeling of Blood Flow for Coronary Artery Bypass Graft Surgery. Ann Biomed Eng 40, 2228–2242 (2012). https://doi.org/10.1007/s10439-012-0579-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-012-0579-3