Abstract
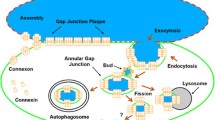
Gap junctions are a unique type of intercellular channels that connect the cytoplasm of adjoining cells. Each gap junction channel is comprised of two hemichannels or connexons and each connexon is formed by the aggregation of six protein subunits known as connexins. Gap junction channels allow the intercellular passage of small (< 1.5 kDa) molecules and regulate essential processes during development and differentiation. However, their role in cell survival and cell death is poorly understood. We review experimental data that support the hypothesis that gap junction channels may propagate cell death and survival modulating signals. In addition, we explore the hypothesis that hemichannels (or unapposed connexons) might be used as a paracrine conduit to spread factors that modulate the fate of the surrounding cells. Finally, direct signal transduction activity of connexins in cell death and survival pathways is addressed.
Similar content being viewed by others
References
Hengartner MO. The biochemistry of apoptosis. Nature 2000; 407: 770–776.
D’Herde K, De Prest B, Mussche S, et al. Ultrastructural localization of cytochrome c in apoptosis demonstrates mitochondrial heterogeneity. Cell Death Differ 2000; 7: 331–337.
Krysko DV, Roels F, Leybaert L, D’Herde K. Mitochondrial transmembrane potential changes support the concept of mitochondrial heterogeneity during apoptosis. J Histochem Cytochem 2001; 49: 1277–1284.
Saelens X, Festjens N, Vande Walle L, van Gurp M, van Loo G, Vandenabeele P. Toxic proteins released from mitochondria in cell death. Oncogene 2004; 23: 2861–2874.
Kanno Y, Loewenstein WR. Intercellular Diffusion. Science 1964; 143: 959–960.
Dewey MM, Barr L. A Study of the Structure and Distribution of the Nexus. J Cell Biol 1964; 23: 553–585.
Revel JP, Karnovsky MJ. Hexagonal array of subunits in intercellular junctions of the mouse heart and liver. J Cell Biol 1967; 33: C7–C12.
Phelan P, Starich TA. Innexins get into the gap. Bioessays 2001; 23: 388–396.
Willecke K, Eiberger J, Degen J, et al. Structural and functional diversity of connexin genes in the mouse and human genome. Biol Chem 2002; 383: 725–737.
Laird DW, Puranam KL, Revel JP. Turnover and phosphorylation dynamics of connexin43 gap junction protein in cultured cardiac myocytes. Biochem J 1991; 273(Pt 1): 67–72.
Goodenough DA, Goliger JA, Paul DL. Connexins, connexons, and intercellular communication. Annu Rev Biochem 1996; 65: 475–502.
Munari-Silem Y, Rousset B. Gap junction-mediated cell-to-cell communication in endocrine glands–molecular and functional aspects: A review. Eur J Endocrinol 1996; 135: 251–264.
Trosko JE, Ruch RJ. Cell-cell communication in carcinogenesis. Front Biosci 1998; 3: d208–d236.
Bruzzone R, White TW, Paul DL. Connections with connexins: the molecular basis of direct intercellular signaling. Eur J Biochem 1996; 238: 1–27.
Bruzzone R, White TW, Goodenough DA. The cellular Internet: On-line with connexins. Bioessays 1996; 18: 709–718.
Musil LS, Goodenough DA. Multisubunit assembly of an integral plasma membrane channel protein, gap junction connexin43, occurs after exit from the ER. Cell 1993; 74: 1065–1077.
Martin PE, Blundell G, Ahmad S, Errington RJ, Evans WH. Multiple pathways in the trafficking and assembly of connexin 26, 32 and 43 into gap junction intercellular communication channels. J Cell Sci 2001; 114: 3845–3855.
Bukauskas FF, Verselis VK. Gap junction channel gating. Biochim Biophys Acta 2004; 1662: 42–60.
White TW, Bruzzone R, Paul DL. The connexin family of intercellular channel forming proteins. Kidney Int 1995; 48: 1148–1157.
Kumar NM, Gilula NB. The gap junction communication channel. Cell 1996; 84: 381–388.
Lampe PD, Lau AF. The effects of connexin phosphorylation on gap junctional communication. Int J Biochem Cell Biol 2004; 36: 1171–1186.
Yamasaki H, Naus CC. Role of connexin genes in growth control. Carcinogenesis 1996; 17: 1199–1213.
Moorby C, Patel M. Dual functions for connexins: Cx43 regulates growth independently of gap junction formation. Exp Cell Res 2001; 271: 238–248.
Mesnil M, Krutovskikh V, Piccoli C, et al. Negative growth control of HeLa cells by connexin genes: Connexin species specificity. Cancer Res 1995; 55: 629–639.
Freeman SM, Abboud CN, Whartenby KA, et al. The “bystander effect”: Tumor regression when a fraction of the tumor mass is genetically modified. Cancer Res 1993; 53: 5274–5283.
Dilber MS, Abedi MR, Christensson B, et al. Gap junctions promote the bystander effect of herpes simplex virus thymidine kinase in vivo. Cancer Res 1997; 57: 1523–1528.
Sanson M, Marcaud V, Robin E, Valery C, Sturtz F, Zalc B. Connexin 43-mediated bystander effect in two rat glioma cell models. Cancer Gene Ther 2002; 9: 149–155.
Cirenei N, Colombo BM, Mesnil M, Benedetti S, Yamasaki H, Finocchiaro G. In vitro and in vivo effects of retrovirus-mediated transfer of the connexin 43 gene in malignant gliomas: consequences for HSVtk/GCV anticancer gene therapy. Gene Ther 1998; 5: 1221–1226.
Bai S, Du L, Liu W, Whittle IR, He L. Tentative novel mechanism of the bystander effect in glioma gene therapy with HSV-TK/GCV system. Biochem Biophys Res Commun 1999; 259: 455–459.
Burrows FJ, Gore M, Smiley WR, et al. Purified herpes simplex virus thymidine kinase retroviral particles: III. Characterization of bystander killing mechanisms in transfected tumor cells. Cancer Gene Ther 2002; 9: 87–95.
Wilson MR, Close TW, Trosko JE. Cell population dynamics (apoptosis, mitosis, and cell-cell communication) during disruption of homeostasis. Exp Cell Res 2000; 254: 257– 268.
Krysko DV, Mussche S, Leybaert L, D’Herde K. Gap junctional communication and connexin43 expression in relation to apoptotic cell death and survival of granulosa cells. J Histochem Cytochem 2004; 52: 1199–1207.
Cotrina ML, Kang J, Lin JH, et al. Astrocytic gap junctions remain open during ischemic conditions. J Neurosci 1998; 18: 2520–2537.
Kalvelyte A, Imbrasaite A, Bukauskiene A, Verselis VK, Bukauskas FF. Connexins and apoptotic transformation. Biochem Pharmacol 2003; 66: 1661–1672.
Krutovskikh VA, Piccoli C, Yamasaki H. Gap junction intercellular communication propagates cell death in cancerous cells. Oncogene 2002; 21: 1989–1999.
Wilson JW, Pritchard DM, Hickman JA, Potten CS. Radiation-induced p53 and p21WAF-1/CIP1 expression in the murine intestinal epithelium: apoptosis and cell cycle arrest. Am J Pathol 1998; 153: 899–909.
Hendry JH, Potten CS. Comments on the paper: Cell survival in irradiated microcolonies: How influential are the neighbours? Int J Radiat Biol 1998; 73: 575–577.
Reznikov K, Kolesnikova L, Pramanik A, et al. Clustering of apoptotic cells via bystander killing by peroxides. Faseb J 2000; 14: 1754–1764.
Sai K, Kang KS, Hirose A, Hasegawa R, Trosko JE, Inoue T. Inhibition of apoptosis by pentachlorophenol in v-myc-transfected rat liver epithelial cells: Relation to down-regulation of gap junctional intercellular communication. Cancer Lett 2001; 173: 163–174.
Trosko JE, Chang CC. Mechanism of up-regulated gap junctional intercellular communication during chemoprevention and chemotherapy of cancer. Mutat Res 2001; 480–481: 219–229.
Contreras JE, Sanchez HA, Veliz LP, Bukauskas FF, Bennett MV, Saez JC. Role of connexin-based gap junction channels and hemichannels in ischemia-induced cell death in nervous tissue. Brain Res Brain Res Rev 2004; 47: 290–303.
Frantseva MV, Kokarovtseva L, Naus CG, Carlen PL, MacFabe D, Perez Velazquez JL. Specific gap junctions enhance the neuronal vulnerability to brain traumatic injury. J Neurosci 2002; 22: 644–653.
Naus CC, Ozog MA, Bechberger JF, Nakase T. A neuroprotective role for gap junctions. Cell Commun Adhes 2001; 8: 325–328.
Frantseva MV, Kokarovtseva L, Perez Velazquez JL. Ischemia-induced brain damage depends on specific gap-junctional coupling. J Cereb Blood Flow Metab 2002; 22: 453–462.
Rawanduzy A, Hansen A, Hansen TW, Nedergaard M. Effective reduction of infarct volume by gap junction blockade in a rodent model of stroke. J Neurosurg 1997; 87: 916–920.
Rami A, Volkmann T, Winckler J. Effective reduction of neuronal death by inhibiting gap junctional intercellular communication in a rodent model of global transient cerebral ischemia. Exp Neurol 2001; 170: 297–304.
Saito R, Graf R, Hubel K, Fujita T, Rosner G, Heiss WD. Reduction of infarct volume by halothane: Effect on cerebral blood flow or perifocal spreading depression-like depolarizations. J Cereb Blood Flow Metab 1997; 17: 857–864.
Berridge MJ, Bootman MD, Lipp P. Calcium—A life and death signal. Nature 1998; 395: 645–648.
Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol 2000; 1: 11–21.
Orrenius S, Zhivotovsky B, Nicotera P. Regulation of cell death: The calcium-apoptosis link. Nat Rev Mol Cell Biol 2003; 4: 552–565.
Felzen B, Shilkrut M, Less H, et al. Fas (CD95/Apo-1)-mediated damage to ventricular myocytes induced by cytotoxic T lymphocytes from perforin-deficient mice: a major role for inositol 1,4,5-trisphosphate. Circ Res 1998; 82: 438–450.
Mutoh T, Kumano T, Nakagawa H, Kuriyama M. Role of tyrosine phosphorylation of phospholipase C gamma1 in the signaling pathway of HMG-CoA reductase inhibitor-induced cell death of L6 myoblasts. FEBS Lett 1999; 446: 91–94.
Amsterdam A, Keren-Tal I, Aharoni D. Cross-talk between cAMP and p53-generated signals in induction of differentiation and apoptosis in steroidogenic granulosa cells. Steroids 1996; 61: 252–256.
Lerner A, Kim DH, Lee R. The cAMP signaling pathway as a therapeutic target in lymphoid malignancies. Leuk Lymphoma 2000; 37: 39–51.
Takadera T, Shiraishi Y, Ohyashiki T. Prostaglandin E2 induced caspase-dependent apoptosis possibly through activation of EP2 receptors in cultured hippocampal neurons. Neurochem Int 2004; 45: 713–719.
Ferrari D, Los M, Bauer MK, Vandenabeele P, Wesselborg S, Schulze-Osthoff K. P2Z purinoreceptor ligation induces activation of caspases with distinct roles in apoptotic and necrotic alterations of cell death. FEBS Lett 1999; 447: 71–75.
Schrier SM, Florea BI, Mulder GJ, Nagelkerke JF, AP IJ. Apoptosis induced by extracellular ATP in the mouse neuroblastoma cell line N1E-115: Studies on involvement of P2 receptors and adenosine. Biochem Pharmacol 2002; 63: 1119–1126.
Seite P, Ruchaud S, Hillion J, et al. Ectopic expression of Bcl-2 switches over nuclear signalling for cAMP-induced apoptosis to granulocytic differentiation. Cell Death Differ 2000; 7: 1081–1089.
Schulte G, Fredholm BB. Signalling from adenosine receptors to mitogen-activated protein kinases. Cell Signal 2003; 15: 813–827.
Conti M. Specificity of the cyclic adenosine 3′,5′-monophosphate signal in granulosa cell function. Biol Reprod 2002; 67: 1653–1661.
Han PJ, Shukla S, Subramanian PS, Hoffman PN. Cyclic AMP elevates tubulin expression without increasing intrinsic axon growth capacity. Exp Neurol 2004; 189: 293–302.
Lin JH, Weigel H, Cotrina ML, et al. Gap-junction-mediated propagation and amplification of cell injury. Nat Neurosci 1998; 1: 494–500.
Peters O, Schipke CG, Hashimoto Y, Kettenmann H. Different mechanisms promote astrocyte Ca2+ waves and spreading depression in the mouse neocortex. J Neurosci 2003; 23: 9888–9896.
Theis M, Jauch R, Zhuo L, et al. Accelerated hippocampal spreading depression and enhanced locomotory activity in mice with astrocyte-directed inactivation of connexin43. J Neurosci 2003; 23: 766–776.
Braet K, Vandamme W, Martin PE, Evans WH, Leybaert L. Photoliberating inositol-1,4,5-trisphosphate triggers ATP release that is blocked by the connexin mimetic peptide gap 26. Cell Calcium 2003; 33: 37–48.
Churchill GC, Louis CF. Roles of Ca2+, inositol trisphosphate and cyclic ADP-ribose in mediating intercellular Ca2+ signaling in sheep lens cells. J Cell Sci 1998; 111(Pt 9): 1217– 1225.
Allbritton NL, Meyer T, Stryer L. Range of messenger action of calcium ion and inositol 1,4,5-trisphosphate. Science 1992; 258: 1812–1815.
Sneyd J, Wetton BT, Charles AC, Sanderson MJ. Intercellular calcium waves mediated by diffusion of inositol trisphosphate: A two-dimensional model. Am J Physiol 1995; 268: C1537–1545.
Lazrak A, Peracchia C. Gap junction gating sensitivity to physiological internal calcium regardless of pH in Novikoff hepatoma cells. Biophys J 1993; 65: 2002–2012.
Saez JC, Connor JA, Spray DC, Bennett MV. Hepatocyte gap junctions are permeable to the second messenger, inositol 1,4,5-trisphosphate, and to calcium ions. Proc Natl Acad Sci USA 1989; 86: 2708–2712.
Niessen H, Harz H, Bedner P, Kramer K, Willecke K. Selective permeability of different connexin channels to the second messenger inositol 1,4,5-trisphosphate. J Cell Sci 2000; 113(Pt 8): 1365–1372.
Patterson RL, Boehning D, Snyder SH. Inositol 1,4,5-trisphosphate receptors as signal integrators. Annu Rev Biochem 2004; 73: 437–465.
Takata M, Homma Y, Kurosaki T. Requirement of phospholipase C-gamma 2 activation in surface immunoglobulin M-induced B cell apoptosis. J Exp Med 1995; 182: 907–914.
Szalai G, Krishnamurthy R, Hajnoczky G. Apoptosis driven by IP(3)-linked mitochondrial calcium signals. Embo J 1999; 18: 6349–6361.
Chen R, Valencia I, Zhong F, et al. Bcl-2 functionally interacts with inositol 1,4,5-trisphosphate receptors to regulate calcium release from the ER in response to inositol 1,4,5-trisphosphate. J Cell Biol 2004; 166: 193–203.
Boehning D, Patterson RL, Sedaghat L, Glebova NO, Kurosaki T, Snyder SH. Cytochrome c binds to inositol (1,4,5) trisphosphate receptors, amplifying calcium-dependent apoptosis. Nat Cell Biol 2003; 5: 1051–1061.
Assefa Z, Bultynck G, Szlufcik K, et al. Caspase-3-induced truncation of type 1 inositol trisphosphate receptor accelerates apoptotic cell death and induces inositol trisphosphate-independent calcium release during apoptosis. J Biol Chem 2004; 279: 43227–43236.
Lawrence TS, Beers WH, Gilula NB. Transmission of hormonal stimulation by cell-to-cell communication. Nature 1978; 272: 501–506.
Murray SA, Fletcher WH. Hormone-induced intercellular signal transfer dissociates cyclic AMP-dependent protein kinase. J Cell Biol 1984; 98: 1710–1719.
Bedner P, Niessen H, Odermatt B, Willecke K, Harz H. A method to determine the relative cAMP permeability of connexin channels. Exp Cell Res 2003; 291: 25–35.
Solares J, Garcia-Dorado D, Oliveras J, et al. Contraction band necrosis at the lateral borders of the area at risk in reperfused infarcts. Observations in a pig model of in situ coronary occlusion. Virchows Arch 1995; 426: 393–399.
Ruiz-Meana M, Garcia-Dorado D, Hofstaetter B, Piper HM, Soler-Soler J. Propagation of cardiomyocyte hypercontracture by passage of Na(+) through gap junctions. Circ Res 1999; 85: 280–287.
Garcia-Dorado D, Inserte J, Ruiz-Meana M, et al. Gap junction uncoupler heptanol prevents cell-to-cell progression of hypercontracture and limits necrosis during myocardial reperfusion. Circulation 1997; 96: 3579–3586.
Rose CR, Ransom BR. Gap junctions equalize intracellular Na+ concentration in astrocytes. Glia 1997; 20: 299–307.
Blanc EM, Bruce-Keller AJ, Mattson MP. Astrocytic gap junctional communication decreases neuronal vulnerability to oxidative stress-induced disruption of Ca2+ homeostasis and cell death. J Neurochem 1998; 70: 958–970.
Ozog MA, Siushansian R, Naus CC. Blocked gap junctional coupling increases glutamate-induced neurotoxicity in neuron-astrocyte co-cultures. J Neuropathol Exp Neurol 2002; 61: 132–141.
Siushansian R, Bechberger JF, Cechetto DF, Hachinski VC, Naus CC. Connexin43 null mutation increases infarct size after stroke. J Comp Neurol 2001; 440: 387–394.
Nakase T, Sohl G, Theis M, Willecke K, Naus CC. Increased apoptosis and inflammation after focal brain ischemia in mice lacking connexin43 in astrocytes. Am J Pathol 2004; 164: 2067–2075.
SiuYi Leung D, Unsicker K, Reuss B. Gap junctions modulate survival-promoting effects of fibroblast growth factor-2 on cultured midbrain dopaminergic neurons. Mol Cell Neurosci 2001; 18: 44–55.
Cheng A, Tang H, Cai J, et al. Gap junctional communication is required to maintain mouse cortical neural progenitor cells in a proliferative state. Dev Biol 2004; 272: 203–216.
Tazuke SI, Schulz C, Gilboa L, et al. A germline-specific gap junction protein required for survival of differentiating early germ cells. Development 2002; 129: 2529–2539.
Mesnil M, Krutovskikh V, Omori Y, Yamasaki H. Role of blocked gap junctional intercellular communication in non-genotoxic carcinogenesis. Toxicol Lett 1995; 82–83: 701– 706.
Goldberg GS, Moreno AP, Lampe PD. Gap junctions between cells expressing connexin 43 or 32 show inverse permselectivity to adenosine and ATP. J Biol Chem 2002; 277: 36725–36730.
White TW. Nonredundant gap junction functions. News Physiol Sci 2003; 18: 95–99.
Goodenough DA, Paul DL. Beyond the gap: Functions of unpaired connexon channels. Nat Rev Mol Cell Biol 2003; 4: 285–294.
Ebihara L. New roles for connexons. News Physiol Sci 2003; 18: 100–103.
Bennett MV, Contreras JE, Bukauskas FF, Saez JC. New roles for astrocytes: gap junction hemichannels have something to communicate. Trends Neurosci 2003; 26: 610–617.
Plotkin LI, Manolagas SC, Bellido T. Transduction of cell survival signals by connexin-43 hemichannels. J Biol Chem 2002; 277: 8648–8657.
Hur KC, Shim JE, Johnson RG. A potential role for cx43-hemichannels in staurosporin-induced apoptosis. Cell Commun Adhes 2003; 10: 271–277.
Cotrina ML, Lin JH, Alves-Rodrigues A, et al. Connexins regulate calcium signaling by controlling ATP release. Proc Natl Acad Sci USA 1998; 95: 15735–15740.
Stout CE, Costantin JL, Naus CC, Charles AC. Intercellular calcium signaling in astrocytes via ATP release through connexin hemichannels. J Biol Chem 2002; 277: 10482–10488.
Braet K, Aspeslagh S, Vandamme W, et al. Pharmacological sensitivity of ATP release triggered by photoliberation of inositol-1,4,5-trisphosphate and zero extracellular calcium in brain endothelial cells. J Cell Physiol 2003; 197: 205–213.
Ye ZC, Wyeth MS, Baltan-Tekkok S, Ransom BR. Functional hemichannels in astrocytes: a novel mechanism of glutamate release. J Neurosci 2003; 23: 3588–3596.
Bruzzone S, Guida L, Zocchi E, Franco L, De Flora A. Connexin 43 hemi channels mediate Ca2+-regulated transmembrane NAD+ fluxes in intact cells. Faseb J 2001; 15: 10–12.
Zipfel GJ, Babcock DJ, Lee JM, Choi DW. Neuronal apoptosis after CNS injury: the roles of glutamate and calcium. J Neurotrauma 2000; 17: 857–869.
Tsukimoto M, Harada H, Ikari A, Takagi K. Involvement of chloride in apoptotic cell death induced by activation of ATP-sensitive P2X7 purinoceptor. J Biol Chem 2004.
Braet K, Mabilde C, Cabooter L, Rapp G, Leybaert L. Electroporation loading and photoactivation of caged InsP3: tools to investigate the relation between cellular ATP release in response to intracellular InsP3 elevation. J Neurosci Methods 2004; 132: 81–89.
Nicotera P, Leist M, Ferrando-May E. Intracellular ATP, a switch in the decision between apoptosis and necrosis. Toxicol Lett 1998; 102–103: 139–142.
Huang RP, Fan Y, Hossain MZ, Peng A, Zeng ZL, Boynton AL. Reversion of the neoplastic phenotype of human glioblastoma cells by connexin 43 (cx43). Cancer Res 1998; 58: 5089–5096.
Qin H, Shao Q, Curtis H, et al. Retroviral delivery of connexin genes to human breast tumor cells inhibits in vivo tumor growth by a mechanism that is independent of significant gap junctional intercellular communication. J Biol Chem 2002; 277: 29132–29138.
Huang RP, Hossain MZ, Huang R, Gano J, Fan Y, Boynton AL. Connexin 43 (cx43) enhances chemotherapy-induced apoptosis in human glioblastoma cells. Int J Cancer 2001; 92: 130–138.
Dang X, Doble BW, Kardami E. The carboxy-tail of connexin-43 localizes to the nucleus and inhibits cell growth. Mol Cell Biochem 2003; 242: 35–38.
de Feijter AW, Matesic DF, Ruch RJ, Guan X, Chang CC, Trosko JE. Localization and function of the connexin 43 gap-junction protein in normal and various oncogene-expressing rat liver epithelial cells. Mol Carcinog 1996; 16: 203– 212.
Kanemitsu MY, Loo LW, Simon S, Lau AF, Eckhart W. Tyrosine phosphorylation of connexin 43 by v-Src is mediated by SH2 and SH3 domain interactions. J Biol Chem 1997; 272: 22824–22831.
Loo LW, Kanemitsu MY, Lau AF. In vivo association of pp60v-src and the gap-junction protein connexin 43 in v-src-transformed fibroblasts. Mol Carcinog 1999; 25: 187–195.
Guerrier A, Fonlupt P, Morand I, et al. Gap junctions and cell polarity: connexin32 and connexin43 expressed in polarized thyroid epithelial cells assemble into separate gap junctions, which are located in distinct regions of the lateral plasma membrane domain. J Cell Sci 1995; 108(Pt 7): 2609–2617.
Lin JH, Yang J, Liu S, et al. Connexin mediates gap junction-independent resistance to cellular injury. J Neurosci 2003; 23: 430–441.
Iacobas DA, Urban-Maldonado M, Iacobas S, Scemes E, Spray DC. Array analysis of gene expression in connexin-43 null astrocytes. Physiol Genomics 2003; 15: 177–190.
Bao X, Chen Y, Reuss L, Altenberg GA. Functional expression in Xenopus oocytes of gap-junctional hemichannels formed by a cysteine-less connexin 43. J Biol Chem 2004; 279: 9689–9692.
Leybaert L, Braet K, Vandamme W, Cabooter L, Martin PE, Evans WH. Connexin channels, connexin mimetic peptides and ATP release. Cell Commun Adhes 2003; 10: 251–257.
Wong CW, Christen T, Kwak BR. Connexins in leukocytes: Shuttling messages? Cardiovasc Res 2004; 62: 357–367.
Ruchaud S, Lanotte M. cAMP and ‘death signals’ in a myeloid leukaemia cell: From membrane receptors to nuclear responses: a review. Biochem Soc Trans 1997; 25: 410–415.
Sasson R, Amsterdam A. Stimulation of apoptosis in human granulosa cells from in vitro fertilization patients and its prevention by dexamethasone: involvement of cell contact and bcl-2 expression. J Clin Endocrinol Metab 2002; 87: 3441–3451.
Author information
Authors and Affiliations
Corresponding author
Additional information
These authors share senior authorship.
This study was supported by Ghent University GOA grant no. 12050502.
Rights and permissions
About this article
Cite this article
Krysko, D.V., Leybaert, L., Vandenabeele, P. et al. Gap junctions and the propagation of cell survival and cell death signals. Apoptosis 10, 459–469 (2005). https://doi.org/10.1007/s10495-005-1875-2
Issue Date:
DOI: https://doi.org/10.1007/s10495-005-1875-2