Abstract
HeLa and HCT116 cells respond differentially to sorbitol, an osmolyte able to induce hypertonic stress. In these models, sorbitol promoted the phenotypic manifestations of early apoptosis followed by complete loss of viability in a time-, dose-, and cell type-specific fashion, by eliciting distinct yet partially overlapping molecular pathways. In HCT116 but not in HeLa cells, sorbitol caused the mitochondrial release of the caspase-independent death effector AIF, whereas in both cell lines cytochrome c was retained in mitochondria. Despite cytochrome c retention, HeLa cells exhibited the progressive activation of caspase-3, presumably due to the prior activation of caspase-8. Accordingly, caspase inhibition prevented sorbitol-induced killing in HeLa, but only partially in HCT116 cells. Both the knock-out of Bax in HCT116 cells and the knock-down of Bax in A549 cells by RNA interference reduced the AIF release and/or the mitochondrial alterations. While the knock-down of Bcl-2/Bcl-XL sensitized to sorbitol-induced killing, overexpression of a Bcl-2 variant that specifically localizes to mitochondria (but not of the wild-type nor of a endoplasmic reticulum-targeted form) strongly inhibited sorbitol effects. Thus, hyperosmotic stress kills cells by triggering different molecular pathways, which converge at mitochondria where pro- and anti-apoptotic members of the Bcl-2 family exert their control.
Similar content being viewed by others
Introduction
The best characterized and the most prominent pathways leading to apoptosis are called the extrinsic and intrinsic pathways. In the extrinsic pathway (also known as “death receptor pathway”), apoptosis is triggered by the ligand-induced activation of death receptors at the cell surface. Such death receptors include the tumor necrosis factor (TNF) receptor-1, CD95/Fas (the receptor of CD95L/FasL), as well as the TNF-related apoptosis inducing ligand (TRAIL) receptors-1 and -2 [1]. Upon binding of their ligands, death receptors recruit the adapter molecule FADD (Fasassociating death domain-containing protein) within the death-inducing signaling complex (DISC). Oligomerized FADD binds initiator caspases-8 and -10, in turn causing their dimerization and activation [2]. In the intrinsic pathway (also called “mitochondrial pathway”), apoptosis results from an intracellular cascade of events in which mitochondrial permeabilization plays a crucial role [3, 4]. The two pathways are interconnected at multiple levels, but the best characterized link is represented by the BH3-only protein Bid, which can be proteolitically activated by caspase-8 [2]. In its truncated active form (tBid), Bid translocates at mitochondria where it promotes membrane permeabilization, for instance by activating other pro-apoptotic members of the Bcl-2 family (e.g. Bax, Bak) [5].
Both routes to apoptotic death can be divided at least in three distinct phases: initiation, integration/decision and execution/degradation [6]. The initiation phase is highly heterogeneous and depends on the nature of the death-inducing signal, be it an extrinsic one (the ligation of a death receptor) or an intrinsic one (which may affect any cellular organelle including the nucleus, the endoplasmic reticulum (ER), lysosomes or mitochondria). The integration/decision phase involves the near-to-simultaneous activation of caspases and mitochondrial death effectors in a complex molecular interplay. During this phase the “decision to die” is taken and the “point-of-no-return” is trespassed. The execution/degradation phase, which is essentially a post-mortem process, is common to distinct types of apoptosis, meaning that the morphological and biochemical alterations that accompany late-stage apoptosis are independent of the initiating stimulus. Both the extrinsic and the intrinsic routes to apoptosis ultimately lead to cell shrinkage, chromatin condensation, nuclear fragmentation (which is frequently accompanied by internucleosomal DNA fragmentation), blebbing and phosphatidylserine exposure on the surface of the plasma membrane [7].
Most cell death in vertebrates proceeds via the intrinsic or mitochondrial pathway of apoptosis [8]. Here, the executioner caspases (including caspase-3) are cleaved and activated by the initiator caspase-9, which is activated by multimerization on the adapter molecule apoptosis-protease activating factor 1 (APAF-1) within a multiprotein complex called “apoptosome”. APAF-1 pre-exists in the cytosol as a monomer, and its activation depends on the presence of cytochrome c (Cyt c) and ATP/dATP [9]. The release of Cyt c, which normally resides only in the mitochondrial intermembrane space (IMS) where it functions as an electron shuttle in the respiratory chain, is rate-limiting for the generation of the apoptosome [10]. Hence, mitochondrial membrane permeabilization (MMP) is the critical event responsible for caspase activation in the intrinsic pathway. MMP can even commit a cell to die when caspases are not activated. This “caspase-independent death” [11, 12] can occur because of an irreversible loss of mitochondrial function (often accompanied by an intense production of reactive oxygen species, i.e. ROS) as well as because of the mitochondrial release of caspase-independent death effectors including apoptosis-inducing factor (AIF) [13], endonuclease G (EndoG), and others [11, 12].
Hyperosmotic stress is one particular condition that can lead to cell death. For instance, hyperosmotic stress play an important role in the pathology of the ischemic heart muscle, where the rapidmobilization of osmolytes occurring upon ischemia promotes a sudden increase in local osmolarity [14]. Interestingly, in cultured cardiomyocytes, the activation of the NF-κB pathway has been reported to mediate caspase-9 activation and eventually cell death as a consequence of sorbitol-induced hypertonic stress [15]. Hyperosmotic stress reportedly elicit numerous signal transduction pathways, and several papers insist on the fact that different osmolytes share the ability to trigger the extrinsic pathway of apoptosis, by activation of the CD95/FAS death receptor system [16, 17], the TNF-α death receptor system [18] or perhaps by interrupting trophic signals delivered by growth factor receptors [19]. Other physiological responses that are modulated by osmolarity and that may induce apoptosis include the acidification of endosomal compartments [20] as well as the degradation of cyclin-dependent kinases [21]. However, the implication of mitochondria in osmolyte-induced apoptosis has not yet been addressed in detail, in the mammalian system.
Here, we addressed the question to what extent mitochondria might contribute to apoptosis induction by hyperosmotic stress, as mimicked by sorbitol administration to cultured human tumor cells. Our results point that hypertonic stress is able to induce characteristic mitochondrial alterations involved in caspase-dependent and caspase-independent cell death, including ROS generation, in a cell type-specific fashion. Moreover, we demonstrate that members of the Bcl-2 family control osmolyte-induced apoptosis at the mitochondrial level.
Materials and methods
Cell lines, culture and treatments
Derivatives of the HCT116 cell line (parental and Bax-/-) were a generous gift of Dr. B. Vogelstein [22] and routinely maintained in McCoy's 5A medium supplemented with 10% heat-inactivated fetal calf serum (FCS). HeLa cells were grown in DMEM (glucose 4,5 g/L) containing L-glutamine and 110 mg/L sodium pyruvate, supplemented with 10% FCS and 10 mM HEPES buffer. A549 were grown in F12-K medium containing L-glutamine, supplemented with 10% FCS, 100 units/ml penicillin G sodium and 100 µg/ml streptomycin sulfate. Rat-1 fibroblasts stably transfected with an empty control vector (CMV) or with a plasmid encoding Bcl-2, either in wild-type configuration (WT) or fused to peptides which allow the targeting to mitochondria (Acta) or to endoplasmic reticulum (Cb5) [23], were kindly provided by Dr. D. Andrews, and cultured in DMEM (glucose 4,5 g/L) containing L-glutamine and 110 mg/L sodium pyruvate, supplemented with 10% FCS, 10 mM HEPES buffer, 100 units/ml penicillin G sodium and 100 µg/ml streptomycin sulfate. All media and supplements for cell culture were purchased from Gibco-Invitrogen (Carlsbad, USA).
30–100 × 103 cells were seeded in 12-well plates and grown for 24 h prior to treatment with D-sorbitol (Sigma-Aldrich, St. Louis, USA) at concentrations varying from 0 to 300 mM (HeLa and HCT116 cells) of from 0 to 1 M (A549 cells) in the presence or absence of the pan-caspase inhibitor N-benzyloxycarbonyl-Val-Ala-Asp.fluoromethylketone (Z-VAD-fmk, purchased from Bachem, Bubendorf, Switzerland) at the concentration of 50 µM or of the p53 inhibitor cyclic pifithrin-α (Sigma-Aldrich) at the concentration of 30 µM. Sorbitol treatments lasted up to a maximum of 48 h. As positive control for caspase-3 activation, cells were treated with 2 µM staurosporine (Sigma-Aldrich) for 6 h. All experiments were repeated at least two times with similar results.
Transfections and siRNAs
HeLa and HCT116 cells were transiently transfected with an empty control vector (CMV) or with a plasmid encoding Bcl-2, either in wild-type configuration (WT) or fused to peptides which allow the targeting to mitochondria (Acta) or to endoplasmic reticulum (Cb5) [23]. Liposome-mediated transfection was performed in 24-well plates by using Lipofectamine™ 2000 Transfection Reagent (Invitrogen), according to the manufacturer instructions. After 24 h, cells were subjected to sorbitol treatment prior to cytofluorometric determination of apoptosis-associated alterations.
The down-regulation of Bak and Bax was performed with siRNAs (Hs_BAK1_5 and Hs_BAX_10 HP Validated siRNAs, respectively) purchased from Qiagen (Hilden, Germany). Knock-down of Bcl-2, Bcl-XL, p53 and the voltage-dependent anion channel 1 (VDAC1) was performed with siRNAs purchased from Sigma-Proligo (The Woodlands, USA). Bcl-2 sense 5′-GCUGCACCUGACGCCCUUCTT-3′, antisense 5′-GAAGGGCGUCAGGUGCAGCTT-3′ [24]; Bcl-XL sense 5′-CAGGGACAGCAUAUCAGAGTT-3′, antisense 5′-CUCUGAUAUGCUGUCCCUGTT-3′ [24]; p53 sense 5′-GACUCCAGUGGUAAUCUACTT-3′, antisense 5′-GUAGAUUACCACUGGAGUCTT-3′ [25]; VDAC1 sense 5′-GUACGGCCUGACGUUUACATT-3′, antisense 5′-UGUAAACGUCAGGCCGUACTT-3′. As a control, an siRNA with an “unrelated”, irrelevant sequence was used (“UNR” sense 5′-GCCGGUAUGCCGGUUAAGUTT-3′, antisense 5′-ACUUAACCGGCAUACCGGCTT-3′). A549 cells were reverse-transfected at the time of plating by means of the HiPerFect transfection reagent (Qiagen), as follows. Transfection complexes were prepared in 100 µl serum-free, antibiotic-free F12-K by mixing 8 µl of HiPerFect transfection reagent with 40 pmol of siRNA and let stand at room temperature for 20–30 min, to allow for efficient complex formation. Transfection complexes were then mixed with 700 µl of complete medium (as detailed above) in which 30–40 × 103 cells had been previously resuspended. Finally, the resulting suspension was seeded as a whole in a single well of a 12-well plate. To allow for complete or near-to-complete protein down-regulation, transfected cells were cultured for 48 h prior to sorbitol treatment.
Cytofluorometric analysis and immunofluorescence
According to previously reported protocols [26], cells were labeled with the following fluorochromes to determine apoptosis-associated changes: propidium iodide (PI, 1 µg/ml, Sigma-Aldrich) for viability, 3,3′dihexiloxalocarbocyanine iodide (DiOC6(3), 40 nM, Molecular Probes-Invitrogen) for ΔΨm dissipation, annexin V-FITC (Molecular Probes-Invitrogen) for assessment of phosphatidylserine (PS) exposure. Hydroethidine (HE, 4 µM, Molecular Probes-Invitrogen) was employed to determine superoxide anion generation, as previously described [27]. In summary, HE is a reduced non-fluorescent precursor that, in the presence of ROS (and more precisely of superoxide anion species), is readily oxidized intracellularly into the fluorescent compound ethidium bromide, which binds to DNA and can be detected by virtue of its red fluorescence. Cytofluorometric analyses were performed by means of a FACS Scan (Becton Dickinson, San Jose, USA). Data were statistically evaluated using Cell Quest software (Becton Dickinson).
Cells were fixed with paraformaldehyde (4% w/v) and subsequently stained with antibodies for the detection of AIF (for HCT116 cells: rabbit polyclonal IgG anti-AIF internal domain, cat no AB16501; Chemicon International, Temecula, USA; for HeLa cells: goat polyclonal IgG AIF (D-20), cat. no SC-9416; Santa Cruz Biotechnology, Santa Cruz, USA), Bcl-2 (mouse monoclonal IgG Bcl-2 (100), cat. no SC-509; Santa Cruz Biotechnology), cytochrome c (rabbit polyclonal IgG cytochrome c (H-104), cat. no SC-7179; Santa Cruz Biotechnology, Santa Cruz, USA) or HSP-60 (mouse monoclonal anti-heat shock protein 60 (clone LK1), cat no H 4149, Sigma-Aldrich). Nuclei were labeled with 10 µg/ml Hoechst 33342 (Molecular Probes-Invitrogen). According to the specific double staining, primary antibodies were revealed either with anti-mouse, anti-rabbit or anti-goat IgG conjugated to either Alexa 488 (green) or Alexa 568 (red) from Molecular Probes-Invitrogen. Fluorescence microscopy assessments were performed with a Leica IRE2 microscope equipped with a Leica DC300F camera.
Analysis of protein expression
Protein samples of HeLa, HCT116 or A549 cells were prepared in lysis buffer, following standard established protocols. Aliquots of the extracted proteins (approx. 50 µg/lane) were separated according to molecular weight on a mono-dimensional 12% SDS-PAGE then subjected to immunoblots using antibodies specific for active caspase-8 (mouse monoclonal IgG anti cleaved caspase-8 (Asp384) (11G10), cat no 9748; Cell Signaling Technology, Danvers, USA) Bak (rabbit polyclonal serum Bak, cat. no, 556396; PharMingen, San Diego, USA), Bax (rabbit polyclonal IgG Bax (N-20), cat. no SC-493; Santa Cruz Biotechnology), Bcl-2 (mouse monoclonal IgG Bcl-2 (100), cat. no SC-509; Santa Cruz Biotechnology), Bcl-X (rabbit polyclonal IgG Bcl-X (Ab-1) (201-216), cat no PC67; Calbiochem, San Diego, USA), GAPDH (mouse monoclonal IgG anti-glyceraldehyde-3-phosphate dehydrogenase (6C5), cat no MAB274; Chemicon International, Temecula, USA), p53 (mouse monoclonal IgG p53 (DO-1), cat no SC-126; Santa Cruz Biotechnology), total caspase-3 (rabbit polyclonal anti caspase-3 (Asp175), cat no 9662; Cell Signaling Technology) or VDAC1 (rabbit polyclonal IgG anti-porin (Ab-5), cat no PC548; Calbiochem). Membranes were then incubated with secondary goat anti-mouse (cat no 1010-05) or anti-rabbit (cat. no 4010-05) IgG conjugated to horseradish peroxidase (SouthernBiotech, Birmingham, USA) prior to revelation by means of ECL Detection Kit (Amersham Pharmacia, Pittsburgh, USA) and Hyperfilm x-ray films (Amersham Pharmacia).
Results and discussion
Cell-type specific response to hyperosmotic stress
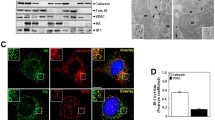
Human tumor cells, including the HCT116 colon carcinoma and the HeLa cervical carcinoma cell lines, manifest the classical features of apoptosis with different sensitivity, when treated with high concentrations of the osmolyte D-sorbitol. Thus, HeLa cells exhibited rapid and massive apoptosis-associated alterations, including the dissipation of the mitochondrial transmembrane potential (ΔΨm) (Fig. 1(a, b)), the exposure of phosphatidylserine (PS) on the outer leaflet of the plasma membrane (Fig. 1(a, b)) and an increased generation of reactive-oxygen species (ROS) (Fig. 1(c)), before losing viability. In addition, HeLa cells manifested the proteolytic maturation of caspase-3, as indicated by the progressive degradation of the inactive pro-caspase-3 in parallel with the accumulation of a ∼17 kDa fragment corresponding to the heavy chain of active caspase-3 (Fig. 1(d)). Caspase-3 activation was preceded by the activation of caspase-8, which reportedly occurs within the extrinsic pathway of apoptosis [2], witnessed by the early accumulation of the light chain (∼10 kDa) of the active caspase-8.
Hyperosmotic stress-induced apoptosis in HeLa cells. HeLa cells were treated for up to 24 h with 0 (control), 25, 50, 100, 200 or 300 mM sorbitol, then submitted to cytofluorometric analysis of mitochondrial membrane potential (DiOC6(3) staining), PS exposure (Annexin V staining) and viability (PI staining). (a) Representative dot plots of HeLa cells treated for 24 h with 0, 200 and 300 mM sorbitol. X-axis, DiOC6(3) (upper panels) and Annexin V staining (lower panels); Y-axis, PI staining. (b) Time- and dose-dependent response of HeLa cells to sorbitol. White columns depict the percentage of cells that have a low mitochondrial membrane potential but are still viable (DiOC6(3)low). Black columns report the percentage of cells that have lost the intactness of plasma membrane (PI+). Light grey columns indicate the percentage of cells that have exposed PS on the outer leaflet of the plasma membrane (Annexin V+). Data are mean of triplicate experiments ± SEM. (c) ROS generation. HeLa cells were treated with 300 mM sorbitol for the indicated time, then analyzed at FACS for the detection of ROS. Dark grey columns report the percentage of cells characterized by an intense production of superoxide anion (HE+). Data are mean of duplicate experiments ± SEM. (d) Caspase activation upon sorbitol treatment. HeLa cells were treated with 300 mM sorbitol or with 2 µM staurosporine (STS, positive control for caspase-3 activation). After the indicated time, the cells lysates were run on a mono-dimensional SDS-PAGE, and analyzed by immunoblotting with antibodies specific for caspase-3, caspase-8 and GAPDH (loading control). Caspase-3 activation coincides with a reduction in the intensity of the pro-caspase band (35 kDa) paralleled by an increase of the bands corresponding to the active fragments (19 and 17 kDa). Caspase-8 activation results in the appearance of a band corresponding to the p10 active fragment. For additional details please refer to the section “Materials and methods”
As compared to HeLa cells, HCT116 cells were more resistant to sorbitol treatment, as indicated by two color staining with the ΔΨm-sensitive dye DiOC6(3) and the vital dye propidium iodide (PI) as well as by reduced PS exposure (Fig. 2(a, b)). This correlated with a lower percentage of HCT116 cells, as compared to HeLa, that overproduced ROS after exposure to sorbitol (Fig. 2(c)). Moreover, HCT116 cells did not manifest any sign of caspase activation upon treatment with sorbitol (Fig. 2(d)), pointing to cell type-specific differences in the lethal process. Nonetheless, the mitochondrial changes induced by sorbitol occurred in a concentration and time-dependent fashion, in both HeLa and HCT116 cells, though to different extents (Figs. 1(b) and 2(b)).
Hyperosmotic stress-induced apoptosis in HCT116 cells. WT HCT116 cells were treated with 0 (control), 25, 50, 100, 200 or 300 mM sorbitol for up to 24 h, then subjected to cytofluorometric measurement of mitochondrial membrane potential (DiOC6(3) staining), PS exposure (Annexin V staining) and viability (PI staining). (a) Typical dot plots of HCT116 cells treated with 0, 200 and 300 mM sorbitol for 24 h. X-axis, DiOC6(3) (upper panels) and Annexin V staining (lower panels); Y-axis, PI staining. (b) Time- and dose-dependent response of WT HCT116 cells to sorbitol. White columns illustrate the percentage of cells which have dissipated the mitochondrial membrane potential but are still viable (DiOC6(3)low). The percentage of cells with disrupted plasma membrane (PI+) is reported by black columns. Light grey columns represent the percentage of cells with PS exposed on the outer leaflet of the plasma membrane (Annexin V+). Data are mean of triplicate experiments ± SEM. (c) ROS generation. WT HCT116 cells were treated for the indicated time with 300 mM sorbitol, stained with HE, and then subjected to cytofluorometric analysis for the detection of ROS. Dark grey columns illustrate the percentage of cells which overproduced superoxide anion (HE+). Data are mean of duplicate experiments ± SEM. (d) Caspase activation upon sorbitol treatment. WT HCT116 cells were treated with 300 mM sorbitol or with 2 µM staurosporine (STS, positive control for caspase-3 activation) for the indicated time, followed by protein purification, SDS-PAGE and immunoblot detection of caspase-3, caspase-8 and GAPDH (loading control). For more detailed information please see also “Materials and methods”
To gain insights into the mechanism of ΔΨm dissipation, cells were exposed to 300 mM sorbitol in the presence of the p53 inhibitor cyclic pifithrin-α or the pan-caspase inhibitor Z-VAD-fmk. As assessed by FACS analysis of ΔΨm, PS exposure and viability, pifithrin-α failed to protect HeLa (Fig. 3(a)) and HCT116 cells (Fig. 3(b)) from sorbitol-induced cell death, and rather sensitized HCT116 cells to the lethal effects of hypertonic stress (Fig. 3(b)). Z-VAD-fmk was able to prevent the ΔΨm dissipation, PS exposure and loss of viability in HeLa cells (Fig. 3(a)), but was only partially effective (especially concerning the loss of viability, as assessed by PI positivity) in HCT116 cells (Fig. 3(b)). This correlated with caspase-3 and caspase-8 activation in HeLa but not in HCT116 cells, and may be linked to the differential sensitivity demonstrated by these cell lines to hyperosmotic stress. Taken altogether, these results indicate that p53 is not required for the mitochondrial changes induced by hyperosmolarity and that the involvement of caspases is cell-type specific.
Roles of caspases, p53 and Bax in hyperosmotic stress-induced apoptosis. HeLa (a) and WT HCT116 (b) cells were treated for 12 (a) or 24 (b) h with 0 (control) or 300 mM sorbitol, in the presence or not of the pan-caspase inhibitor Z-VAD-fmk or of the p53 inhibitor cyclic pifithrin-α, then analyzed at FACS for mitochondrial membrane potential (DiOC6(3) staining), PS exposure (Annexin V staining) and viability (PI staining). (c) WT and Bax-/- HCT116 cells were treated for 12 or 24 h with 300 mM sorbitol then submitted to cytofluorometric analysis of mitochondrial membrane potential (DiOC6(3) staining) and viability (PI staining). (a–c) White columns report the percentage of cells that have a low mitochondrial membrane potential but are still viable (DiOC6(3)low). Black columns specify the percentage of cells which have lost the integrity of plasma membrane (PI+). (a, b) Light grey columns depict the percentage of cells which have exposed PS on the outer leaflet of the plasma membrane (Annexin V+). Data are mean of triplicate experiments ± SEM. (d) To check for down-regulation, total proteins were purified from WT and Bax-/- HCT116 cells, run on mono-dimensional SDS-PAGE, then subjected to immunoblot analysis with antibodies against Bax and GAPDH (loading control). For further details please refer to the section “Materials and methods”
As an additional internal control, we took advantage of HCT116 cells that were either sufficient for Bax (wild type, WT) or lacked Bax expression as a result of homologous recombination (Bax-/-). Of note, in the same conditions in which cyclic pifithrin-α and Z-VAD-fmk failed to exert cytoprotective effects, the removal of Bax did reduce (though partially) the sorbitol-triggered ΔΨm dissipation (Fig. 3(c)), meaning that the ΔΨm loss can be modulated by proteins such as Bax that control the mitochondrial phase of apoptosis. Altogether, these data indicate that caspases are activated by hyperosmotic stress in a cell type-specific fashion, thus supporting the notion that caspase activation is not an universal prerequisite for the ΔΨm loss that defines the “pointof-no-return” of the lethal process [7].
AIF and Cyt c subcellular localization upon hyperosmotic stress
Since Bax was (at least partially) required for sorbitol-induced ΔΨm loss, we suspected that specific mitochondrial alterations might accompany the cell death triggered by hyperosmotic stress. Thus, we performed immunofluorescence studies on cells that had been treated with sorbitol, to determine the subcellular localization of two proteins that under physiological conditions reside in the mitochondrial intermembrane space (IMS), namely AIF and Cyt c. As shown in Fig. 4(a), WT HCT116 cells manifested a clear relocation of AIF from a cytoplasmic punctuate distribution (which closely resembles that of the mitochondrial marker protein HSP-60) in controls, to a more diffuse pattern all over the cell (including the nucleus) after sorbitol administration. In contrast, no similar mitochondrio-nuclear translocation of AIF was observed when Bax-/- HCT116 cells were treated with sorbitol (Fig. 4(a)). In sharp contrast with AIF, Cyt c remained in a clearly mitochondrial localization, co-staining with HSP-60, both in WT and Bax-/- HCT116 cells treated with sorbitol (Fig. 4(b)). These results pointed to a differential release mechanism for AIF and Cyt c. Indeed, it has been previously reported that AIF can exit mitochondria before Cyt c, for example when the latter is retained by electrostatic interactions with cardiolipin, a lipid of the inner mitochondrial membrane [28].
Subcellular localization of apoptosis-inducing factor (AIF) and cytochrome c (Cyt c) in HCT116 cells undergoing hyperosmotic stress-induced apoptosis. (a, b) WT and Bax-/- HCT116 cells were treated or not with 300 mM sorbitol for 2 h, followed by immunofluorescence staining with antibodies specific for AIF (a) or Cyt c (b) and the 60 kDa heat-shock protein (HSP-60, which stains mitochondria). Diffuse AIF staining indicates that AIF is released from mitochondria upon sorbitol treatment in WT, but not in Bax-/-, HCT116 cells (a). At the same time point, Cyt c is retained in mitochondria of sorbitol-treated HCT116 cells (WT and Bax-/-) (b). White bars represent picture scale (10 µm)
In an attempt to confirm these results in another cell line, we were surprised to find that in HeLa cells sorbitol failed to induce the release of both AIF (Fig. 5(a)) and Cyt c (Fig. 5(b)). Interestingly, the mitochondrial retention of Cyt c (Fig. 4(b)) correlated with the failure of HCT116 cells to activate caspase-3 (Fig. 2(d)), while the same did not hold true in HeLa cells, where the progressive activation of caspase-3 (Fig. 1(d)) occured independently from the liberation of Cyt c (Fig. 5(b)). In HeLa cells, the proteolytic maturation of caspase-3 may occur following the activation of caspase-8 (Fig. 1(d)). In this context, it has previously been described that different osmolytes elicit the extrinsic pathway of apoptosis [16–18], thus leading to the activation of caspase-8 [2], which reportedly is able to cleave and activate pro-caspase-3 in a direct fashion [29]. In summary, hitherto unexplained cell type-specific differences must be taken into account when studying stress-elicited mitochondrial alterations. As a possibility, hyperosmotic stress may trigger distinct proapoptotic mechanism in different cell types, either by acting on mitochondria to promote AIF release (as in HCT116 cells) or by activating the caspase cascade of the extrinsic pathway (as in HeLa cells). The involvement of mitochondria, be it direct or not, appears to be the unifying element of alterations induced by hypertonic stress.
Apoptosis-inducing factor (AIF) and cytochrome c (Cyt c) retention in HeLa cells undergoing hyperosmotic stress-induced apoptosis. (a, b) HeLa cells were treated with 0 (control) or 300 mM sorbitol for 2 h, then stained with Hoechst 33342 and antibodies specific for AIF (a) or Cyt c (b) and the 60 kDa heat-shock protein (HSP-60, which co-localizes with mitochondria). Mitochondria of HeLa cells retained both AIF (a) and Cyt c (b) after 2 h of treatment with sorbitol. The same holds true for treatments up to 6 h (data not shown). White bars represent picture scale (10 µm).
Bcl-2 family proteins control cell death induced by hyperosmotic stress
The data shown above indicate that osmotic stress is able to induce specific changes in the mitochondrial function that are regulated, at least partially, by pro-apoptotic Bcl-2 proteins such as Bax. We therefore took advantage of a non-small cell lung cancer model, A549 cells, that we are optimizing for use in high-throughput screening assays. A549 cells underwent sorbitol-induced cell death similarly to HeLa or HCT116 cells, yet were even more resistant to hypertonic stress (Fig. 6(a)). In this experimental system, the siRNA-mediated knock-down of several members of the Bcl-2 family (Fig. 6(b)), significantly influenced sorbitol-induced killing. For instance, the knock-down of Bax (but not that of Bak) diminished sorbitol-induced killing, while the down-regulation of Bcl-2 or Bcl-XL enhanced cell death induced by osmotic shock (Fig. 6(c)). Notably, the knockdown of either p53 and of the voltage-dependent anion channel 1 (VDAC1, a major component of the mitochondrial permeability transition pore complex) [30, 31] had negligible effects on sorbitol-induced apoptosis (Fig. 6(c)). This correlates with the lack of cytoprotection conferred by cyclic pifithrin-α to HeLa and HCT116 cells treated with sorbitol (Fig. 3(a, b)).
Involvement of the Bcl-2 protein family in hyperosmotic stressinduced death of A549 cells. (a) A549 cells were treated with the indicated dose of sorbitol for 6 h, followed by the cytofluorometric assessment of mitochondrial membrane potential (DiOC6(3) staining) and viability (PI staining). White columns illustrate the percentage of cells with a low mitochondrial membrane potential but still viable (DiOC6(3)low). Black columns indicate the percentage of cells with disrupted plasma membrane (PI+). Data are mean of duplicate experiments ± SEM. (b, c) A549 cells were transfected with empty liposomes (Mock) or with siRNAs targeting Bax, Bak, Bcl-2, Bcl-XL, p53, the voltage-dependent anion channel 1 (VDAC1) or an irrelevant “unrelated” control (UNR). (b) To check for the effects of siRNAs, total proteins were purified from transfected A549 cells at different time points (12, 24 or 48 h), separated according to molecular weight on mono-dimensional SDS-PAGE, and finally analyzed by immunoblotting with the indicated antibodies. Antibodies specific for Mcl-1 and Bcl-XL or for GAPDH were employed as loading controls. (c) A549 cells transfected for 48 h with the indicated siRNAs were treated with 600 mM sorbitol for additional 6 h, then analyzed at FACS for mitochondrial membrane potential (DiOC6(3) staining) and viability (PI staining). White columns depict the percentage of cells that have dissipated the mitochondrial membrane potential but are still viable (DiOC6(3)low). The percentage of cells with disrupted plasma membrane (PI+) is illustrated by black columns. Data are mean of duplicate experiments ± SEM. Dashed lines indicate the range of statistical insignificance from control cells (p > 0.05, ± 3 SEM). For additional details please see also “Materials and methods”
To confirm the role of Bcl-2 in the pro-apoptotic cascades elicited by hyperosmotic stress, we transiently transfected HeLa and HCT116 cells with plasmids encoding either WT Bcl-2 or Bcl-2 variants that selectively localize to mitochondria (Bcl-2 Acta) or to the endoplasmic reticulum (Bcl-2 Cb5) [23]. In HeLa, Bcl-2 Acta was able to reduce all the apoptosis-associated alterations induced by sorbitol, including ΔΨm dissipation, PS exposure and loss of viability (Fig. 7(a)). In the same cells, WT Bcl-2 was effective only against the loss of ΔΨm, but did not decrease PS exposure nor PI positivity, and Bcl-2 Cb5 had no effects on sorbitol-induced alterations (Fig. 7(a)). In HCT116 cells, WT Bcl-2 and Bcl-2 Cb5 were totally ineffective and only the Bcl-2 isoform targeted to mitochondria (Acta) provided some level of cytoprotection against hypertonic stress-induced apoptosis, by partially preventing ΔΨm dissipation and loss of viability, but not PS exposure (Fig. 7(b)). Again, these data point to the existence of cell type-specific responses to hyperosmotic stress.
Bcl-2 targeted to mitochondria protects HeLa and HCT116 cells from hyperosmotic stress-induced cell death. HeLa (a) and HCT116 (b) cells were transiently transfected with an empty control vector (CMV) or with a plasmid expressing Bcl-2, either in its wild-type configuration (WT) or fused to mitochondria- and endoplasmic reticulum-targeting peptides (Acta and Cb5, respectively). After 24 h, cells were treated with 0 (control) or 300 mM sorbitol for additional 6, 12 or 24 h, then stained for the cytofluorometric determination of mitochondrial membrane potential (DiOC6(3) staining), PS exposure (Annexin V staining) and viability (PI staining). White columns report the percentage of cells that have a low mitochondrial membrane potential but are still viable (DiOC6(3)low). Black columns illustrate the percentage of cells with disrupted plasma membrane (PI+). Light grey columns depict the percentage of cells characterized by PS on the outer leaflet of the plasma membrane (Annexin V+). Data are mean of duplicate experiments ± SEM. For more detailed information please refer to the section “Materials and methods”
Finally, the cytoprotective effect of Bcl-2 overexpression was explored in a fibroblast cell line (Rat-1) that was stably transfected with the abovementioned constructs encoding human WT Bcl-2, or Bcl-2 variants that selectively localize to mitochondria (Bcl-2 Acta) and hence co-localize with the mitochondrial marker HSP-60 or to the endoplasmic reticulum (Bcl-2 Cb5), which do not co-localize with HSP-60 (Fig. 8(a, b)) [23]. Similarly to HeLa cells, Rat-1 fibroblasts responded to sorbitol with the progressive activation of caspase-3, a dose-dependent dissipation of ΔΨm and a reduction of viability (Fig. 8(c, d, e)). In this system, the overexpression of Bcl-2 Acta efficiently prevented the proteolytic maturation of caspase-3, while WT Bcl-2 and Bcl-2 Cb5 only retarded its activation (Fig. 8(c)). Of note, both WT Bcl-2 and Bcl-2 Acta (but not Bcl-2 Cb5) reduced the mitochondrial perturbation and the loss of cellular viability induced by sorbitol (Fig. 8(d, e)). Taken altogether, these results demonstrate that mitochondrion-targeted Bcl-2 specifically reduces the mitochondriotoxic and cytotoxic effects of hyperosmotic stress.
Bcl-2 targeted to mitochondria protects Rat-1 fibroblasts from hyperosmotic stress-induced apoptosis. (a–e) Rat-1 fibroblasts were transfected with an empty control vector (CMV) or with a plasmid expressing Bcl-2 in its wild-type configuration (WT) or fused to peptides targeting it to mitochondria (Acta) or to endoplasmic reticulum (Cb5). (a) Rat-1 cells transfected with the abovementioned constructs were submitted to immunofluorescence staining with antibodies specific for Bcl-2 and the 60 kDa heat-shock protein (HSP-60, which colocalizes with mitochondria), to ensure the correct localization of the Bcl-2 fusion proteins. (b) Proteinswere extracted from Rat-1 fibroblasts transfected with the abovementioned plasmids, separated according to molecular weight on mono-dimensional SDS-PAGE, then analyzed by immunoblotting with antibodies specific for Bcl-2 and GAPDH (loading control). (c–e) Transfected Rat-1 fibroblasts were treated with 300 mM sorbitol for the indicated time then subjected to protein extraction for subsequent immunoblot (c) or to cytofluorometric analysis of mitochondrial membrane potential (DiOC6(3) staining) and viability (PI staining) (d–e). (c) After the treatment with 300 mM sorbitol for the indicated time, proteins were extracted, run on mono-dimensional SDS-PAGE, then analyzed by immunoblotting with antibodies specific for total caspase-3 and GAPDH (loading control). Caspase-3 activation corresponds to the progressive accumulation of the 17 kDa active caspase-3 fragment in parallel with a decrease of the pro-caspase-3 band (approx. 35 kDa). Only when Bcl-2 is targeted to mitochondria, sorbitol-induced caspase-3 activation is efficiently prevented. (d) Representative dot plots obtained after 6 h of treatment with 0 (control) or 300 mM sorbitol. X-axis, DiOC6(3) staining; Y-axis, PI staining. (e) Kinetic response of Rat-1 fibroblasts to sorbitol treatment. White columns depict the percentage of cells which have dissipated the mitochondrial membrane potential but are still viable (DiOC6(3)low). The percentage of cells which have lost the integrity of plasma membrane (PI+) is illustrated by black columns. Data are mean of triplicate experiments ± SEM. For further details please see the section “Materials and methods”
Conclusion
The data presented in this article strongly suggest that mitochondria play a major role in the lethal response to hyperosmotic stress. First, cells exposed to hyperosmotic stress manifest characteristic mitochondrial dysfunctions, in particular a loss of the mitochondrial transmembrane potential (ΔΨm), an increased generation of reactive oxygen species (ROS), and an at least partial permeabilization of the outer mitochondrial membrane, leading to the release of AIF (which can act as a caspase-independent death effector), in a cell type-specific fashion. Second, such mitochondrial changes occurred in some cell types (HCT116) in a caspase-independent fashion, because they were not accompanied by caspase activation and were not prevented by the pan-caspase inhibitor Z-VAD-fmk. In contrast, in other cell types (HeLa), the mitochondrial alterations occurred after the osmolyte-induced activation of caspases-8 and -3. Third, proteins belonging to the Bcl-2 family, which are known to exert a major effect on the apoptosis-associated functional and structural impairment of mitochondria, strongly influenced sorbitol-induced apoptosis. As a formal prove of the mitochondrial implication in the lethal process, we found that a mitochondrion-targeted Bcl-2 mutant could confer strong cytoprotection against cell death induced by hypertonic stress.
The differential sensitivity to cell death of the cell lines used in the present study, as well as the cell type-specific mitochondrial changes that we observed in response to hyperosmotic stress, may be related to differences in their proliferative and/or metabolic status [32]. Several observations suggest that proliferation and/or metabolic activity play a role in determining to which extent cells activate specific pathways to regulate cell function upon hypertonic stress [33, 34]. Moreover, it has been reported that changes occurring within the cell as a result of active metabolism/cell division likely promote the same alterations in intracellular water homeostasis resulting from the exposure to extracellular hypertonicity [35]. Thus, the osmotic stress response pathway appears to mediate cellular adaptation not only to overt extracellular hyperosmotic stress, but also to other processes that affect intracellular water homeostasis.
Although the data shown here strongly implicate mitochondria in sorbitol-induced apoptosis, a number of conundra remain to be resolved. Thus, it is not clear which signals act upstream of mitochondria to elicit their permeabilization. Although we did not explicitly address the implication of the extrinsic pathway in sorbitol-induced apoptosis, our data point to an active involvement of the death receptor system, but only in HeLa, not in HCT116 cells. Caspases-8 and -3 were not activated following hyperosmotic stress in HCT116 cells, arguing against a major participation of the death receptor-triggered caspase activation cascade in this model. Future studies will have to clarify at which stage of the apoptotic cascade the cell type-specific pathways elicited by hypertonic stress converge, and which are the molecular players that determine cell type-specific difference in the response to hyperosmotic stress. For instance, it will be interesting to determine to which extent specific BH3 only proteins from the Bcl-2 family, lipid messengers and perturbations in ion or redox homeostasis contribute to trigger the mitochondrial phase of apoptosis in response to hyperosmotic stress.
Abbreviations
- ΔΨm :
-
mitochondrial transmembrane potential
- AIF:
-
apoptosis-inducing factor
- Cyt c :
-
cytochrome c
- DiOC6(3):
-
3,3′dihexiloxalocarbocyanine iodide
- FACS:
-
fluorescence-activated cell sorter
- FITC:
-
fluorescein isothiocyanate
- GAPDH:
-
glyceraldehyde phosphate dehydrogenase
- HE:
-
hydroethidine
- HSP-60:
-
heat shock protein of 60 kDa
- IMS:
-
mitochondrial intermembrane space
- PI:
-
propidium iodide
- PS:
-
phosphatidylserine
- ROS:
-
reactive oxygen species
- siRNA:
-
small interfering RNA
- VDAC1:
-
voltage-dependent anion channel 1
- WT:
-
wild type
- Z-VAD-fmk:
-
N-benzyloxycarbonyl-Val-Ala-Asp.fluoromethylketone
References
Wajant H (2002) The Fas signaling pathway: more than a paradigm. Science 296:1635–636
Debatin KM, Krammer PH (2004) Death receptors in chemotherapy and cancer. Oncogene 23:2950–966
Ferri KF, Kroemer G (2001) Organelle-specific initiation of cell death pathways. Nat Cell Biol 3:E255–63
Ravagnan L, Roumier T, Kroemer G (2002) Mitochondria, the killer organelles and their weapons. J Cell Physiol 192:131–37
Gonzalvez F, Pariselli F, Dupaigne P, et al (2005) tBid interaction with cardiolipin primarily orchestrates mitochondrial dysfunctions and subsequently activates Bax and Bak. Cell Death Differ 12:614–26
Kroemer G, Zamzami N, Susin SA (1997) Mitochondrial control of apoptosis. Immunol Today 18:44–1
Zamzami N, Susin SA, Marchetti P, et al (1996) Mitochondrial control of nuclear apoptosis. J Exp Med 183:1533–544
Green DR, Kroemer G (2004) The pathophysiology of mitochondrial cell death. Science 305:626–29
Cain K, Bratton SB, Cohen GM (2002) The Apaf-1 apoptosome: a large caspase-activating complex. Biochimie 84:203–14
Garrido C, Galluzzi L, Brunet M, Puig PE, Didelot C, Kroemer G (2006) Mechanisms of cytochrome c release from mitochondria. Cell Death Differ 13:1423–433
Chipuk JE, Green DR (2005) Do inducers of apoptosis trigger caspase-independent cell death? Nat Rev Mol Cell Biol 6:268–75
Kroemer G, Martin SJ (2005) Caspase-independent cell death. Nat Med 11:725–30
Susin SA, Lorenzo HK, Zamzami N, et al (1999) Molecular characterization of mitochondrial apoptosis-inducing factor. Nature 397:441–46
Maldonado C, Cea P, Adasme T, et al (2005) IGF-1 protects cardiac myocytes from hyperosmotic stress-induced apoptosis via CREB. Biochem Biophys Res Commun 336:1112–118
Eisner V, Quiroga C, Criollo A, et al (2006) Hyperosmotic stress activates p65/RelB NFkappaB in cultured cardiomyocytes with dichotomic actions on caspase activation and cell death. FEBS Lett 580:3469–476
Reinehr R, Haussinger D (2006) Hyperosmotic activation of the CD95 death receptor system. Acta Physiol (Oxf) 187:199–03
Reinehr R, Schliess F, Haussinger D (2003) Hyperosmolarity and CD95L trigger CD95/EGF receptor association and tyrosine phosphorylation of CD95 as prerequisites for CD95 membrane trafficking and DISC formation. Faseb J 17:731–33
Lang KS, Fillon S, Schneider D, Rammensee HG, Lang F (2002) Stimulation of TNF alpha expression by hyperosmotic stress. Pflugers Arch 443:798–03
Copp J, Wiley S, Ward MW, Van Der Geer P (2005) Hypertonic shock inhibits growth factor receptor signaling, induces caspase-3 activation, and causes reversible fragmentation of the mitochondrial network. Am J Physiol Cell Physiol 288: C403–15
Reinehr R, Becker S, Braun J, Eberle A, Grether-Beck S, Haussinger D (2006) Endosomal acidification and activation of NADPH oxidase isoforms are upstream events in hyperosmolarity-induced hepatocyte apoptosis. J Biol Chem 281:23150–3166
Tao GZ, Rott LS, Lowe AW, Omary MB (2002) Hyposmotic stress induces cell growth arrest via proteasome activation and cyclin/cyclin-dependent kinase degradation. J Biol Chem 277:19295–9303
Zhang L, Yu J, Park BH, Kinzler KW, Vogelstein B (2000) Role of BAX in the apoptotic response to anticancer agents. Science 290:989–92
Zhu W, Cowie A, Wasfy GW, Penn LZ, Leber B, Andrews DW (1996) Bcl-2 mutants with restricted subcellular location reveal spatially distinct pathways for apoptosis in different cell types. EMBO J 15:4130–141
Jiang M, Milner J (2003) Bcl-2 constitutively suppresses p53-dependent apoptosis in colorectal cancer cells. Genes Dev 17:832–37
Gu J, Zhang L, Swisher SG, Liu J, Roth JA, Fang B (2004) Induction of p53-regulated genes in lung cancer cells: implications of the mechanism for adenoviral p53-mediated apoptosis. Oncogene 23:1300–307
Metivier D, Dallaporta B, Zamzami N, et al (1998) Cytofluorometric detection of mitochondrial alterations in early CD95/Fas/APO-1-triggered apoptosis of Jurkat T lymphoma cells. Comparison of seven mitochondrion-specific fluorochromes. Immunol Lett 61:157–63
Zamzami N, Marchetti P, Castedo M, et al (1995) Sequential reduction of mitochondrial transmembrane potential and generation of reactive oxygen species in early programmed cell death. J Exp Med 182:367–77
Modjtahedi N, Giordanetto F, Madeo F, Kroemer G (2006) Apoptosis-inducing factor: vital and lethal. Trends Cell Biol 16:264–72
Stennicke HR, Jurgensmeier JM, Shin H, et al (1998) Pro-caspase-3 is a major physiologic target of caspase-8. J Biol Chem 273:27084–7090
Zamzami N, Kroemer G (2001) The mitochondrion in apoptosis: how Pandora’s box opens. Nat Rev Mol Cell Biol 2:67–1
Tsujimoto Y, Nakagawa T, Shimizu S (2006) Mitochondrial membrane permeability transition and cell death. Biochim Biophys Acta 1757:1297–300
Ho SN (2006) Intracellular water homeostasis and the mammalian cellular osmotic stress response. J Cell Physiol 206:9–5
Lopez-Rodriguez C, Aramburu J, Rakeman AS, Rao A (1999) NFAT5, a constitutively nuclear NFAT protein that does not cooperate with Fos and Jun. Proc Natl Acad Sci U S A 96:7214–219
Trama J, Lu Q, Hawley RG, Ho SN (2000) The NFAT-related protein NFATL1 (TonEBP/NFAT5) is induced upon T cell activation in a calcineurin-dependent manner. J Immunol 165:4884–894
Ho SN (2003) The role of NFAT5/TonEBP in establishing an optimal intracellular environment. Arch Biochem Biophys 413:151–57
Acknowledgment
Dr. Kroemer is supported by the “Ligue Nationale Contre le Cancer” and the European Commission (RIGHT, Trans-Death, Active p53). Dr. Lavandero is supported by grant no 15010006 of the FONDAP (Fondo de Areas Prioritarias, Fondo Nacional de Desarrollo Cientifico y Tecnologico, CONICYT, Chile). A. Criollo is a recipient of a Ph.D. fellowship from CONICYT, Chile. We also thank the International Collaboration Program ECOS-CONICYT, grant C04B03 (to G.K. and S.L.).
Author information
Authors and Affiliations
Corresponding author
Additional information
A. Criollo and L. Galluzzi contributed equally to this work.
An erratum to this article can be found online at http://dx.doi.org/10.1007/s10495-013-0817-7.
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License ( https://creativecommons.org/licenses/by-nc/2.0 ), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Criollo, A., Galluzzi, L., Chiara Maiuri, M. et al. Mitochondrial control of cell death induced by hyperosmotic stress. Apoptosis 12, 3–18 (2007). https://doi.org/10.1007/s10495-006-0328-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-006-0328-x