Abstract
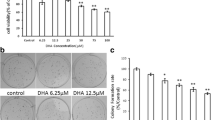
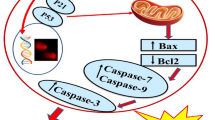
Different agents able to modulate apoptosis have been shown to modify the expression of the MAP-kinase-phosphatase-1 (MKP-1). The expression of this phosphatase has been considered a potential positive prognostic factor in lung cancer, and smoke was shown to reduce the levels of MKP-1 in ferret lung. Our aim was to assess whether the n-3 polyunsaturated fatty acid docosahexaenoic acid (DHA), known to inhibit the growth of several cancer cells mainly inducing apoptosis, may exert pro-apoptotic effect in lung cancer cells by modifying MKP-1 expression. We observed that DHA increased MKP-1 protein and mRNA expression and induced apoptosis in different lung cancer cell lines (mink Mv1Lu adenocarcinoma cells, human A549 adenocarcinoma and human BEN squamous carcinoma cells). We inhibited the pro-apoptotic effect of DHA by treating the cells with the phosphatase inhibitor Na3VO4 or by silencing the MKP-1 gene with the specific siRNA. This finding demonstrated that the induction of apoptosis by DHA involved a phosphatase activity, specifically that of MKP-1. DHA reduced also the levels of the phosphorylated MAP-kinases, especially ERK1/2 and p38. Such an effect was not observed when the MKP-1 gene was silenced. Altogether, the data provide evidence that the DHA-induced overexpression of MKP-1 and the resulting decrease of MAP-kinase phosphorylation by DHA may underlie the pro-apoptotic effect of this fatty acid in lung cancer cells. Moreover, they support the hypothesis that DHA may exert chemopreventive action in lung cancer.






Similar content being viewed by others
References
Calviello G, Serini S, Palozza P (2006) n-3 Polyunsaturated fatty acids as signal transduction modulators and therapeutical agents in cancer. Curr Signal Transduct Ther 1:255–271. doi:10.2174/157436206778226923
Siddiqui RA, Shaikh SR, Sech LA, Yount HR, Stillwell W, Zaloga GP (2004) Omega 3-fatty acids: health benefits and cellular mechanisms of action. Mini Rev Med Chem 4:859–871
Calviello G, Di Nicuolo F, Gragnoli S, Piccioni E, Serini S, Maggiano N et al (2004) n-3 PUFAs reduce VEGF expression in human colon cancer cells modulating the COX-2/PGE2 induced ERK-1 and -2 and HIF-1alpha induction pathway. Carcinogenesis 25:2303–2310. doi:10.1093/carcin/bgh265
Swamy MV, Cooma I, Patlolla JM, Simi B, Reddy BS, Rao CV (2004) Modulation of cyclooxygenase-2 activities by the combined action of celecoxib and decosahexaenoic acid: novel strategies for colon cancer prevention and treatment. Mol Cancer Ther 3:215–221
Liao Z, Mason KA, Milas L (2007) Cyclo-oxygenase-2 and its inhibition in cancer: is there a role? Drugs 67:821–845. doi:10.2165/00003495-200767060-00001
Calviello G, Resci F, Serini S, Piccioni E, Toesca A, Boninsegna A et al (2007) Docosahexaenoic acid induces proteasome-dependent degradation of beta-catenin, down-regulation of survivin and apoptosis in human colorectal cancer cells not expressing COX-2. Carcinogenesis 28:1202–1209. doi:10.1093/carcin/bgl254
Wada T, Penninger JM (2004) Mitogen-activated protein kinases in apoptosis regulation. Oncogene 23:2838–2849. doi:10.1038/sj.onc.1207556
Pervin S, Singh R, Freije WA, Chaudhuri G (2003) MKP-1-induced dephosphorylation of extracellular signal-regulated kinase is essential for triggering nitric oxide-induced apoptosis in human breast cancer cell lines: implications in breast cancer. Cancer Res 63:8853–8860
Kim GS, Choi YK, Song SS, Kim WK, Han BH (2005) MKP-1 contributes to oxidative stress-induced apoptosis via inactivation of ERK1/2 in SH-SY5Y cells. Biochem Biophys Res Commun 338:1732–1738. doi:10.1016/j.bbrc.2005.10.143
Germann A, Dihlmann S, Hergenhahn M, Doeberitz MK, Koesters R (2003) Expression profiling of CC531 colon carcinoma cells reveals similar regulation of beta-catenin target genes by both butyrate and aspirin. Int J Cancer 106:187–197. doi:10.1002/ijc.11215
Xu Q, Konta T, Nakayama K, Furusu A, Moreno-Manzano V, Lucio-Cazana J et al (2004) Cellular defense against H2O2-induced apoptosis via MAP kinase-MKP-1 pathway. Free Radic Biol Med 36:985–993. doi:10.1016/j.freeradbiomed.2004.01.009
Guo YL, Kang B, Williamson JR (1998) Inhibition of the expression of mitogen-activated protein phosphatase-1 potentiates apoptosis induced by tumor necrosis factor-alpha in rat mesangial cells. J Biol Chem 273:10362–10366. doi:10.1074/jbc.273.17.10362
Wahab N, Cox D, Witherden A, Mason RM (2007) Connective tissue growth factor (CTGF) promotes activated mesangial cell survival via up-regulation of mitogen-activated protein kinase phosphatase-1 (MKP-1). Biochem J 406:131–138. doi:10.1042/BJ20061817
Small GW, Shi YY, Higgins LS, Orlowski RZ (2007) Mitogen-activated protein kinase phosphatase-1 is a mediator of breast cancer chemoresistance. Cancer Res 67:4459–4466. doi:10.1158/0008-5472.CAN-06-2644
Loda M, Capodieci P, Mishra R, Yao H, Corless C, Grigioni W et al (1996) Expression of mitogen-activated protein kinase phosphatase-1 in the early phases of human epithelial carcinogenesis. Am J Pathol 149:1553–1564
Manzano RG, Montuenga LM, Dayton M, Dent P, Kinoshita I, Vicent S et al (2002) CL100 expression is down-regulated in advanced epithelial ovarian cancer and its re-expression decreases its malignant potential. Oncogene 21:4435–4447. doi:10.1038/sj.onc.1205542
Tsujita E, Taketomi A, Gion T, Kuroda Y, Endo K, Watanabe A et al (2005) Suppressed MKP-1 is an independent predictor of outcome in patients with hepatocellular carcinoma. Oncology 69:342–347. doi:10.1159/000089766
Vicent S, Garayoa M, Lopez-Picazo JM, Lozano MD, Toledo G, Thunnissen FB et al (2004) Mitogen-activated protein kinase phosphatase-1 is overexpressed in non-small cell lung cancer and is an independent predictor of outcome in patients. Clin Cancer Res 10:3639–3649. doi:10.1158/1078-0432.CCR-03-0771
Liu C, Russell RM, Wang XD (2004) Low dose beta-carotene supplementation of ferrets attenuates smoke-induced lung phosphorylation of JNK, p38 MAPK, and p53 proteins. J Nutr 134:2705–2710
Wang HY, Cheng Z, Malbon CC (2003) Overexpression of mitogen-activated protein kinase phosphatases MKP1, MKP2 in human breast cancer. Cancer Lett 191:229–237. doi:10.1016/S0304-3835(02)00612-2
Kurt RA, Urba WJ, Smith JW, Schoof DD (1998) Peripheral T lymphocytes from women with breast cancer exhibit abnormal protein expression of several signaling molecules. Int J Cancer 78:16–20. doi :10.1002/(SICI)1097-0215(19980925)78:1<16::AID-IJC4>3.0.CO;2-#
Sun H, Charles CH, Lau LF, Tonks NK (1993) MKP-1 (3CH134), an immediate early gene product, is a dual specificity phosphatase that dephosphorylates MAP kinase in vivo. Cell 5:487–493. doi:10.1016/0092-8674(93)90383-2
Misra-Press A, Rim CS, Yao H, Roberson MS, Stork PJ (1995) A novel mitogen-activated protein kinase phosphatase. Structure, expression, and regulation. J Biol Chem 270:14587–14596. doi:10.1074/jbc.270.8.3475
Camps M, Nichols A, Arkinstall S (2000) Dual specificity phosphatases: a gene family for control of MAP kinase function. FASEB J 14:6–16
Franklin CC, Kraft AS (1997) Conditional expression of the mitogen-activated protein kinase (MAPK) phosphatase MKP-1 preferentially inhibits p38 MAPK and stress-activated protein kinase in U937 cells. J Biol Chem 272:16917–16923. doi:10.1074/jbc.272.27.16917
Engelbrecht AM, Engelbrecht P, Genade S, Niesler C, Page C, Smuts M et al (2005) Long-chain polyunsaturated fatty acids protect the heart against ischemia/reperfusion-induced injury via a MAPK dependent pathway. J Mol Cell Cardiol 39:940–954. doi:10.1016/j.yjmcc.2005.08.004
Collett ED, Davidson LA, Fan YY, Lupton JR, Chapkin RS (2001) n-6 and n-3 polyunsaturated fatty acids differentially modulate oncogenic Ras activation in colonocytes. Am J Physiol Cell Physiol 280:C1066–C1075
Denys A, Aires V, Hichami A, Khan NA (2004) Thapsigargin-stimulated MAP kinase phosphorylation via CRAC channels and PLD activation: inhibitory action of docosahexaenoic acid. FEBS Lett 564:177–1782. doi:10.1016/S0014-5793(04)00361-8
Denys A, Hichami A, Khan NA (2002) Eicosapentaenoic acid and docosahexaenoic acid modulate MAP kinase enzyme activity in human T-cells. Mol Cell Biochem 232:143–148. doi:10.1023/A:1014806122510
Denys A, Hichami A, Khan NA (2001) Eicosapentaenoic acid and docosahexaenoic acid modulate MAP kinase (ERK1/ERK2) signaling in human T cells. J Lipid Res 42:2015–2020
Denys A, Hichami A, Maume B, Khan NA (2001) Docosahexaenoic acid modulates phorbol ester-induced activation of extracellular signal-regulated kinases 1 and 2 in NIH/3T3 cells. Lipids 36:813–818. doi:10.1007/s11745-001-0789-2
Yusufi AN, Cheng J, Thompson MA, Walker HJ, Gray CE, Warner GM et al (2003) Differential effects of low-dose docosahexaenoic acid and eicosapentaenoic acid on the regulation of mitogenic signaling pathways in mesangial cells. J Lab Clin Med 141:318–329. doi:10.1016/S0022-2143(03)00005-2
Moon Y, Pestka JJ (2003) Deoxynivalenol-induced mitogen-activated protein kinase phosphorylation and IL6 expression in mice suppressed by fish oil. J Nutr Biochem 14:717–726. doi:10.1016/j.jnutbio.2003.08.009
Diep QN, Touyz RM, Schiffrin EL (2000) Docosahexaenoic acid, a peroxisome proliferator-activated receptor-alpha ligand, induces apoptosis in vascular smooth muscle cells by stimulation of p38 mitogen-activated protein kinase. Hypertension 36:851–855
Xue H, Wan M, Song D, Li Y, Li J (2006) Eicosapentaenoic acid and docosahexaenoic acid modulate mitogen-activated protein kinase activity in endothelium. Vascul Pharmacol 44:434–439. doi:10.1016/j.vph.2006.02.005
Jia Q, Zhou HR, Bennink M, Pestka JJ (2004) Docosahexaenoic acid attenuates mycotoxin-induced immunoglobulin a nephropathy, interleukin-6 transcription, and mitogen-activated protein kinase phosphorylation in mice. J Nutr 134:3343–3349
Narayanan BA (2006) Chemopreventive agents alters global gene expression pattern: predicting their mode of action and targets. Curr Cancer Drug Targets 6:711–727. doi:10.2174/156800906779010218
Pardini RS, Wilson D, Schiff S, Bajo SA, Pierce R (2005) Nutritional intervention with omega-3 Fatty acids in a case of malignant fibrous histiocytoma of the lungs. Nutr Cancer 52:121–129. doi:10.1207/s15327914nc5202_2
Bradley MO, Webb NL, Anthony FH, Devanesan P, Witman PA, Hemamalini S et al (2001) Tumor targeting by covalent conjugation of a natural fatty acid to paclitaxel. Clin Cancer Res 7:3229–3238
Payne M, Ellis P, Dunlop D, Ranson M, Danson S, Schacter L et al (2006) DHA-paclitaxel (Taxoprexin) as first-line treatment in patients with stage IIIB or IV non-small cell lung cancer: report of a phase II open-label multicenter trial. J Thorac Oncol 1:984–990. doi:10.1097/01243894-200611000-00011
Trombetta A, Maggiora M, Martinasso G, Cotogni P, Canuto RA, Muzio G (2007) Arachidonic and docosahexaenoic acids reduce the growth of A549 human lung-tumor cells increasing lipid peroxidation and PPARs. Chem Biol Interact 165:239–250. doi:10.1016/j.cbi.2006.12.014
Tanaka Y, Goto K, Matsumoto Y, Ueoka R (2008) Remarkably high inhibitory effects of docosahexaenoic acid incorporated into hybrid liposomes on the growth of tumor cells along with apoptosis. Int J Pharm 359:264–271. doi:10.1016/j.ijpharm.2008.03.045
Gorman A, McCarthy J, Finucane D, Reville W, Cotter TG (1996) In: Cotter TG, Martin SJ (eds) Techniques in apoptosis. Portland Press Ltd., London, pp 7–9
Calviello G, Di Nicuolo F, Piccioni E, Marcocci ME, Serini S, Maggiano N et al (2003) γ-Tocopheryl quinone induces apoptosis in cancer cells via caspase-9 activation and cytochrome c release. Carcinogenesis 24:42–433. doi:10.1093/carcin/24.3.427
Di Nicuolo F, Serini S, Boninsegna A, Palozza P, Calviello G (2001) Redox regulation of cell proliferation by pyrrolidine dithiocarbamate in murine thymoma cells transplanted in vivo. Free Radic Biol Med 31:1424–1431. doi:10.1016/S0891-5849(01)00714-6
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. doi:10.1016/0003-2697(76)90527-3
Slack DN, Seternes OM, Gabrielsen M, Keyse SM (2001) Distinct binding determinants for ERK2/p38α and JNK map kinases mediate catalytic activation and substrate selectivity of map kinase phosphatase-1. J Biol Chem 276:16491–16500. doi:10.1074/jbc.M010966200
Chen YL, Lin PC, Chen SP, Lin CC, Tsai NM, Cheng YL et al (2007) Activation of NAG-1 via ERK1/2 mitogen-activated protein kinase revealed a isochaihulactone-triggered apoptotic pathway in human lung cancer A549 cells. J Pharmacol Exp Ther 323:746–756. doi:10.1124/jpet.107.126193
Hsiao YC, Kuo WH, Chen PN, Chang HR, Lin TH, Yang WE et al (2007) Flavanone and 2′-OH flavanone inhibit metastasis of lung cancer cells via down-regulation of proteinases activities and MAPK pathway. Chem Biol Interact 167:193–206. doi:10.1016/j.cbi.2007.02.012
Chen KH, Weng MS, Lin JK (2007) Tangeretin suppresses IL-1beta-induced cyclooxygenase (COX)-2 expression through inhibition of p38 MAPK, JNK, and AKT activation in human lung carcinoma cells. Biochem Pharmacol 73:215–227. doi:10.1016/j.bcp. 2006.09.018
Kawai N, Tsujii M, Tsuji S (2002) Cyclooxygenases and colon cancer. Prostaglandins Other Lipid Mediat 68–69:187–196. doi:10.1016/S0090-6980(02)00030-8
Wang H, MacNaughton WK (2005) Overexpressed beta-catenin blocks nitric oxide-induced apoptosis in colonic cancer cells. Cancer Res 65:8604–8607. doi:10.1158/0008-5472.CAN-05-1169
Monjazeb AM, High KP, Connoy A, Hart LS, Koumenis C, Chilton FH (2006) Arachidonic acid-induced gene expression in colon cancer cells. Carcinogenesis 27:1950–1960. doi:10.1093/carcin/bgl023
Dempke W, Rie C, Grothey A, Schmoll HJ (2001) Cyclooxygenase-2: a novel target for cancer chemotherapy? J Cancer Res Clin Oncol 127:411–417. doi:10.1007/s004320000225
Gupta S, Srivastava M, Ahmad N, Sakamoto K, Bostwick DG, Mukhtar H (2001) Lipoxygenase-5 is overexpressed in prostate adenocarcinoma. Cancer 91:737–743
Petkova DK, Clelland C, Ronan J, Pang L, Coulson JM, Lewis S, Knox AJ (2004) Overexpression of cyclooxygenase-2 in non-small cell lung cancer. Respir Med 98:164–172
Aires V, Hichami A, Filomenko R, Plé A, Rébé C, Bettaieb A, Khan NA (2007) Docosahexaenoic acid induces increases in [Ca2+]i via inositol 1,4,5-triphosphate production and activates protein kinase C gamma and -delta via phosphatidylserine binding site: implication in apoptosis in U937 cells. Mol Pharmacol 72:1545–1556
Narayanan BA, Narayanan NK, Reddy BS (2001) Docosahexaenoic acid regulated genes and transcription factors inducing apoptosis in human colon cancer cells. Int J Oncol. 19:1255–1262
Heimli H, Giske C, Naderi S, Drevon CA, Hollung K (2002) Eicosapentaenoic acid promotes apoptosis in Ramos cells via activation of caspase-3 and -9. Lipids 37:797–802
Arita K, Kobuchi H, Utsumi T, Takehara Y, Akiyama J, Horton AA, Utsumi K (2001) Mechanism of apoptosis in HL-60 cells induced by n-3 and n-6 polyunsaturated fatty acids. Biochem Pharmacol 62:82182–82188
Avula CP, Zaman AK, Lawrence R, Fernandes G (1999) Induction of apoptosis and apoptotic mediators in Balb/C splenic lymphocytes by dietary n-3 and n-6 fatty acids. Lipids 34:921–927
Danbara N, Yuri T, Tsujita-Kyutoku M, Sato M, Senzaki H, Takada H, Hada T, Miyazawa T, Okazaki K, Tsubura A (2004) Conjugated docosahexaenoic acid is a potent inducer of cell cycle arrest and apoptosis and inhibits growth of colo 201 human colon cancer cells. Nutr Cancer 50:71–79
Llor X, Pons E, Roca A, Alvarez M, Mañé J, Fernández-Bañares F, Gassull MA (2003) The effects of fish oil, olive oil, oleic acid and linoleic acid on colorectal neoplastic processes. Clin Nutr 22:71–79
Wu M, Harvey KA, Ruzmetov N, Welch ZR, Sech L, Jackson K, Stillwell W, Zaloga GP, Siddiqui RA (2005) Omega-3 polyunsaturated fatty acids attenuate breast cancer growth through activation of a neutral sphingomyelinase-mediated pathway. Int J Cancer 117:34034–34038
Siddiqui RA Jenski LJ, Harvey KA, Wiesehan JD, Stillwell W, Zaloga GP (2003) Cell-cycle arrest in Jurkat leukaemic cells: a possible role for docosahexaenoic acid. Biochem J 371:621–629
Acknowledgement
This work was supported in part by grant D1 2006 to GC from the Catholic University of Sacred Hearth within its program of promotion and diffusion of scientific research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Serini, S., Trombino, S., Oliva, F. et al. Docosahexaenoic acid induces apoptosis in lung cancer cells by increasing MKP-1 and down-regulating p-ERK1/2 and p-p38 expression. Apoptosis 13, 1172–1183 (2008). https://doi.org/10.1007/s10495-008-0246-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-008-0246-1