Abstract
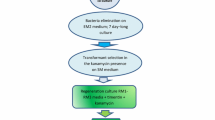
Production of Agrobacterium tumefaciens-mediated transgenic plants, via direct shoot bud organogenesis from leaves of Catharanthus roseus, is reported. A. tumefaciens harbouring the plasmid pBI121 with GUS gene uidA and kanamycin resistance gene nptII was used. Highest transformation efficiency of 1.4 transgenic shoots/responded explant was obtained when pre-plasmolysed leaves, pre-incubated on shoot bud induction medium for 10 days, were subjected to sonication for 30 s prior to transformation. Using a selection medium containing 50 mg kanamycin l−1, transformants grew into micro-shoots and formed roots on a hormone-free half strength MS medium. The transgenic nature of the regenerated plants was confirmed by PCR amplification of uidA gene and GUS histochemical assay.

Similar content being viewed by others
References
Canel C, Lopes-Cardoso MI, Whitmer S, van der Fits L, Pasquali G, van der Heijden R, Hoge JHC, Verpoorte R (1998) Effects of over expression of strictosidine synthase and tryptophan decarboxylase on alkaloid production by cell cultures of Catharanthus roseus. Planta 205:414–419
Choi PS, Kim YD, Choi KM, Chung HJ, Choi DW, Liu JR (2004) Plant regeneration from hairy root cultures transformed by infection with A. rhizogenes in Catharanthus roseus. Plant Cell Rep 22:823–831
Dhandapani M, Kim DH, Hong S (2008) Efficient plant regeneration via somatic embryogenesis and organogenesis from explants of Catharanthus roseus. In Vitro Cell Dev Biol Plant 44:18–25
Di Flore S, Hoppmann V, Fischer R, Schillberg S (2004) Transient gene expression of recombinant terpenoid indole alkaloid enzymes in Catharanthus roseus leaves. Plant Mol Biol Rep 22:15–22
Guirimand G, Burlat V, Oudin A, Lanoue A, St-Pierre B, Courdevault V (2009) Optimization of the transient transformation of Catharanthus roseus cells by particle bombardment and its application to the sub-cellular localization of hydroxymethylbutenyl 4-diphosphate synthase and geraniol 10-hydroxylase. Plant Cell Rep 28:1215–1234
Hooykaas PJJ, Klapwijk PM, Nuti MP, Schilperoort RA, Rorsch A (1977) Transfer of the Agrobacterium tumefaciens Ti-plasmid to avirulent Agrobacteria and to Rhizobium explanta. J Gen Microbiol 98:477–482
Jefferson RA, Kavanagh TA, Bevan MW (1987) GUS fusions: β glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 13:3901–3907
Khanuja SPS, Shasany AK, Darokar MP, Kumar S (1999) Rapid isolation of DNA from dry and fresh samples of plants producing large amounts of secondary metabolites and essential oils. Plant Mol Biol Rep 17:1–7
Mahroug S, Burlat V, St-Pierre B (2007) Cellular and sub-cellular organization of the monoterpenoid indole alkaloid pathway in Catharanthus roseus. Phytochem Rev 6:363–381
Newell CA (2000) Plant transformation technology: developments and applications. Mol Biotechnol 16:53–65
Pietrosiuk A, Furmanowa M, Lata B (2007) Catharanthus roseus: micropropagation and in vitro techniques. Phytochem Rev 6:459–473
Sharma KK, Mathur PB, Thorpe TA (2005) Genetic transformation technology: status and problems. In Vitro Cell Dev Biol Plant 41:102–112
Sretenovic-Rajicic T, Ninkovic S, Miljus-Dukic J, Vinterhalter B, Vinterhalte D (2006) Agrobacterium rhizogenes-mediated transformation of Brassica oleracea var sabauda and B. oleracea var. capitata. Biol Plant 40:525–530
van der Heijden R, Jacobs DJ, Snoeijer W, Hallard D, Verpoorte R (2004) The Catharanthus alkaloids: pharmacognosy and biotechnology. Curr Med Chem 11:1241–1253
Vancanneyt G, Schmidt R, O’Connor-Sanchez A, Willmitzer L, Rocha-Sosa M (1990) Construction of an intron-containing marker gene: splicing of the intron in transgenic plants and its use in monitoring early events in Agrobacterium-mediated plant transformation. Mol Gen Genet 220:245–250
Ya-ping Y, Zhe-Zhi W (2007) Genetic transformation of the medicinal plant Salvia miltiorrhiza by Agrobacterium tumefaciens-mediated method. Plant Cell Tissue Organ Cult 88:175–184
Zarate R, Verpoorte R (2007) Strategies for the genetic modification of the medicinal plant Catharanthus roseus (L.) G. Don. Phytochem Rev 6:475–491
Zhao J, Verpoorte R (2007) Manipulating indole alkaloid production by Catharanthus roseus cell cultures in bioreactors: from biochemical processing to metabolic engineering. Phytochem Rev 6:435–457
Acknowledgments
This work was financially supported by The Council of Scientific and Industrial Research (CSIR), New Delhi (India) through its Network Project COR-02. Authors are also grateful to Dr D.V. Amla, National Botanical Research Institute, Lucknow (India) for providing the gene constructs and to Dr A.K. Gupta and Dr A.K. Shasany, CIMAP, Lucknow for providing the seeds and plant material of C. roseus genotype. The help rendered by Dr A. Mathur in PCR characterization work is also acknowledged. PV also thanks CSIR for awarding a senior research fellowship to her during the course of this investigation. We are also grateful to Director, CIMAP for providing facilities and support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Verma, P., Mathur, A.K. Agrobacterium tumefaciens-mediated transgenic plant production via direct shoot bud organogenesis from pre-plasmolyzed leaf explants of Catharanthus roseus. Biotechnol Lett 33, 1053–1060 (2011). https://doi.org/10.1007/s10529-010-0515-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-010-0515-2