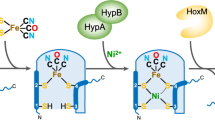
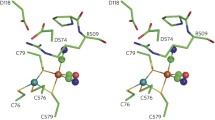
Abstract
Hydrogenases catalyze the reversible oxidation of dihydrogen. Catalysis occurs at bimetallic active sites that contain either nickel and iron or only iron and the nature of these active sites forms the basis of categorizing the enzymes into three classes, the [NiFe]-hydrogenases, the [FeFe]-hydrogenases and the iron sulfur cluster-free [Fe]-hydrogenases. The [NiFe]-hydrogenases and the [FeFe]-hydrogenases are unrelated at the amino acid sequence level but the active sites share the unusual feature of having diatomic ligands associated with the Fe atoms in the these enzymes. Combined structural and spectroscopic studies of [NiFe]-hydrogenases identified these diatomic ligands as CN- and CO groups. Major advances in our understanding of the biosynthesis of these ligands have been achieved primarily through the study of the membrane-associated [NiFe]-hydrogenases of Escherichia coli. A complex biosynthetic machinery is involved in synthesis and attachment of these ligands to the iron atom, insertion of the Fe(CN)2CO group into the apo-hydrogenase, introduction of the nickel atom into the pre-formed active site and ensuring that the holoenzyme is correctly folded prior to delivery to the membrane. Although much remains to be uncovered regarding each of the individual biochemical steps on the pathway to synthesis of a fully functional enzyme, our understanding of the initial steps in CN- synthesis have revealed that it is generated from carbamoyl phosphate. What is becoming increasingly clear is that the metabolic origins of the carbonyl group may be different.






Similar content being viewed by others
References
Adams MW (1990) The structure and mechanism of iron-hydrogenases. Biochem Biophys Acta 1020:115–145
Adams MWW, Hall DO (1979) Purification of membrane-bound hydrogenase of Escherichia coli. Biochem J 183:11–22
Andrews SC, Berks BC, McClay J et al (1997) A 12-cistron Escherichia coli operon (hyf) encoding a putative proton-translocating formate hydrogenlyase system. Microbiology 143:3633–3647
Armstrong FA, Albracht SP (2005) [NiFe]-hydrogenases: spectroscopic and electrochemical definition of reactions and intermediates. Philos Trans A Math Phys Eng Sci 363:937–954
Atanassova A, Zamble DB (2005) Escherichia coli HypA is a zinc metalloprotein with a weak affinity for nickel. J Bacteriol 187:4689–4697
Ballantine SP, Boxer DH (1985) Nickel-containing hydrogenase isoenzymes from anaerobically grown Escherichia coli K-12. J Bacteriol 163:454–459
Ballantine SP, Boxer DH (1986) Isolation and characterisation of a soluble active fragment of hydrogenase isoenzyme 2 from the membranes of anaerobically grown Escherichia coli. Eur J Biochem 156:277–284
Barrett EL, Kwan HS, Macy J (1984) Anaerobiosis, formate, nitrate, and pyrA are involved in the regulation of formate hydrogenlyase in Salmonella typhimurium. J Bacteriol 158:972–977
Blokesch M, Böck A (2002) Maturation of [NiFe]-hydrogenases in Escherichia coli: the HypC cycle. J Mol Biol 324:287–296
Blokesch M, Böck A (2006) Properties of the [NiFe]-hydrogenase maturation protein HypD. FEBS Lett 580:4065–4068
Blokesch M, Magalon A, Böck A (2001) Interplay between the specific chaperone-like proteins HybG and HypC in maturation of hydrogenases 1, 2, and 3 from Escherichia coli. J Bacteriol 183:2817–2822
Blokesch M, Albracht SPJ, Matzanke BF, Drapal N, Böck A (2004a) The complex between hydrogenase-maturation proteins HypC and HypD is an intermediate in the supply of cyanide to the active site iron of [NiFe]-hydrogenases. J Mol Biol 344:155–167
Blokesch M, Paschos A, Bauer A, Reissmann S, Drapal N, Böck A (2004b) Analysis of the transcarbamoylation-dehydration reaction catalyzed by the hydrogenase maturation proteins HypF and HypE. Eur J Biochem 271:3428–3436
Blokesch M, Rohrmoser M, Rode S, Böck A (2004c) HybF, a zinc containing protein involved in NiFe hydrogenase maturation. J Bacteriol 186:2603–2611
Böck A, King PW, Blokesch M, Posewitz MC (2006) Maturation of hydrogenases. Adv Microbiol Physiol 51:1–71
Böhm R, Sauter M, Böck A (1990) Nucleotide sequence and expression of an operon in Escherichia coli coding for formate hydrogenlyase components. Mol Microbiol 4:231–243
Burgdorf T, Lenz O, Buhrke T et al (2005) [NiFe]-hydrogenases of Ralstonia eutropha H16: Modular enzymes for oxygen-tolerant biological hydrogen oxidation. J Mol Microbiol Biotechnol 10:181–196
Butland G, Zhang JW, Yang W et al (2006) Interactions of the Escherichia coli hydrogenase biosynthetic proteins: HybG complex formation. FEBS Lett 580:677–681
Cammack R, Frey M, Robson R (2001) Hydrogen as a fuel: learning from nature. Taylor & Francis, London
DerVartanian ME, Menon NK, Pryzbyla AE, Peck HD Jr, DerVartanian DV (1996) Electron paramagnetic resonance (EPR) studies on hydrogenase-1 (HYD1) purified from a mutant strain (AP6) of Escherichia coli enhanced in HYD1. Biochem Biophys Res Commun 227:211–215
Drapal N, Böck A (1998) Interaction of the hydrogenase accessory protein HypC with HycE, the large subunit of Escherichia coli hydrogenase 3 during enzyme maturation. Biochemistry 37:2941–2948
Dubini A, Sargent F (2004) Assembly of Tat-dependent [NiFe] hydrogenases: identification of precursor-binding accessory proteins. FEBS Lett 549:141–146
Fritsche E, Paschos A, Beisel HG, Böck A, Huber R (1999) Crystal structure of the hydrogenase maturating endodpeptidase HYBD from Escherichia coli. J Mol Biol 288:989–998
Gasper R, Scrima A, Wittinghofer A (2006) Structural insights into HypB, a GTP-binding protein that regulates metal binding. J Biol Chem 281:27492–27502
Graham A (1981) The organisation of hydrogenase in the cytoplasmic membrane of Escherichia coli. Biochem J 197:283–291
Hedderich R, Forzi L (2005) Energy-converting [NiFe] hydrogenases: more than just H2 activation. J Mol Microbiol Biotechnol 10:92–104
Horner DS, Heil B, Happe T, Embley TM (2002) Iron hydrogenses - ancient enzymes in modern eukaryotes. Trends Biochem Sci 27:148–153
Hube M, Blokesch M, Böck A (2002) Network of hydrogenase maturation in Escherichia coli: role of accessory proteins HypA and HybF. J Bacteriol 184:3879–3885
Jack RL, Buchanan G, Dubini A, Hatzixanthis K, Palmer T, Sargent F (2004) Coordinating assembly and export of complex bacterial proteins. EMBO J 23:3962–3972
Jacobi A, Rossmann R, Böck A (1992) The hyp operon gene products are required for the maturation of catalytically active hydrogenase isoenzymes in Escherichia coli. Arch Microbiol 158:444–451
Jiang J, Acunzo A, Koch SA (2001) Chemistry of [FeII(CN)5(CO)]3-: new observations for a 19th century problem. J Am Chem Soc 123:12109–12110
Leach MR, Sandal S, Sun H, Zamble DB (2005) Metal binding activity of the Escherichia coli hydrogenase maturation factor HypB. Biochemistry 44:12229–12238
Lutz S, Jacobi A, Schlensog V, Böhm B, Sawers G, Böck A (1991) Molecular characterisation of an operon (hyp) necessary for the activity of the three hydrogenase isoenzymes in Escherichia coli. Mol Microbiol 5:123–135
Lyon EJ, Shima S, Boecher R et al (2004a) Carbon monoxide as an intrinsic ligand to iron in the active site of the iron-sulfur-cluster-free hydrogenase H2-forming methylenetetrahydromethanopterin dehydrogenase as revealed by infrared spectroscopy. J Am Chem Soc 126:14239–14248
Lyon EJ, Shima S, Buurmann G, Chowdhuri S et al (2004b) UV-A/blue-light inactivation of the ‘metal-free’ hydrogenase (Hmd) from methanogenic archaea. Eur J Biochem 271:195–204
Magalon A, Böck A (2000a) Analysis of the HypC-HycE complex, a key intermediate in the assembly of the metal center of the Escherichia coli hydrogenase 3. J Biol Chem 275:21114–21220
Magalon A, Böck A (2000b) Dissection of the maturation reactions of the [NiFe] hydrogenase 3 from Escherichia coli taking place after nickel incorporation. FEBS Lett 473:254–258
Maier T, Böck A (1996) Generation of active [NiFe] hydrogenase in vitro from a nickel-free precursor form. Biochemistry 35:10089–10093
Maier T, Jacobi A, Sauter M, Böck A (1993) The product of the hypB gene, which is required for nickel incorporation into hydrogenases, is a novel guanine nucleotide-binding protein. J Bacteriol 175:630–635
Maier T, Binder U, Böck A (1996) Analysis of the hydA locus of Escherichia coli: two genes (hydN and hypF) involved in formate and hydrogen metabolism. Arch Microbiol 165:333–341
Mathews RG (1996) One-carbon metabolism. In: Neidhardt FC et al (eds) Escherichia coli and Salmonella: molecular and cellular biology, 2nd edn. ASM Press, pp 600–611
Melis A, Happe T (2001) Hydrogen production: green algae as a source of energy. Plant Physiol 127:740–748
Menon NK, Robbins J, Wendt JC, Shanmugam KT, Przybyla AE (1991) Mutational analysis and characterisation of the Escherichia coli hya operon, which encodes (NiFe) hydrogenase 1. J Bacteriol 173:4851–4861
Menon NK, Chatelus CY, Dervartanian M, Wendt JC, Shanmugam KT, Peck Jr HD, Przybyla AE (1994) Cloning, sequencing, and mutational analysis of the hyb operon encoding Escherichia coli hydrogenase 2. J Bacteriol 176:4416–4423
Nicolet Y, Piras C, Legrand P, Hatchikian EC, Fontecilla-Camps J (1999) Desulfovibrio desulfuricans iron hydrogenase: the structure shows unusual coordination of an active site Fe binuclear center. Structure 7:13–23
Nicolet Y, Cavazza C, Fontecilla-Camps J (2002) Fe-only hydrogenases: structure, function and evolution. J Inorg Biochem 91:1–8
Palmer T, Sargent F, Berks BC (2005). Export of complex cofactor-containing proteins by the bacterial Tat pathway. Trends Microbiol 13:175–180
Paschos A, Glass RS, Böck A (2001) Carbamoyl phosphate requirement for synthesis of the active center of [NiFe]-hydrogenases. FEBS Lett 488:9–12
Paschos A, Bauer A, Zimmermann A, Zehelein E, Böck A (2002) HypF, a carbamoyl phosphate-converting enzyme involved in [NiFe] hydrogenase maturation. J Biol Chem 277:49945–49951
Peters JW (1999) Structure and mechanism of iron-only hydrogenases. Curr Opin Struct Biol 9:670–676
Peters JW, Lanzilotta WN, Lemon BJ, Seefeldt LC (1998) X-ray crystal structure of the Fe-only hydrogenase (Cp1) from Clostridium pasteurianum to 1.8 angstrom resolution. Science 282:1853–1858
Peters JW, Szilagyi RK, Naumov A, Douglas T (2005) A radical solution for the biosynthesis of the H-cluster of hydrogenase. FEBS Lett 580:363–367
Pickett CJ, Vincent KA, Ibrahim SK et al (2004) Synergic binding of carbon monoxide and cyanide to the FeMo cofactor of nitrogenase: relic chemistry of an ancient enzyme? Chem Eur J 10:4770–4776
Pierik AJ, Hulstein M, Hagen WR, Albracht SPJ (1998) A low-spin iron with CN and CO as intrinsic ligands forms the core of the active site in [Fe]-hydrogenases. Eur J Biochem 258:572–578
Pierik AJ, Roseboom W, Happe RP, Bagley KA, Albracht SPJ (1999) Carbon monoxide and cyanide as intrinsic ligands to iron in the active site of [NiFe]-hydrogenases. J Biol Chem 274:3331–3337
Ragsdale SW (2004) Life with carbon monoxide. Crit Rev Biochem Mol Biol 39:165–195
Reissmann S, Hochleitner E, Wang H et al (2003) Taming of a poison: biosynthesis of the [NiFe]-hydrogenase cyanide ligands. Science 299:1067–1070
Rossmann R, Sawers G, Böck A (1991) Mechanism of regulation of the formate-hydrogenlyase pathway by oxygen, nitrate, and pH: definition of the formate regulon. Mol Microbiol 5:2807–2814
Rossmann R, Sauter M, Lottspeich F, Böck A (1994) Maturation of the large subunit (HycE) of hydrogenase 3 of Escherichia coli requires nickel incorporation followed by C-terminal processing at Arg537. Eur J Biochem 220:377–384
Rossmann R, Maier T, Lottspeich F, Böck A (1995) Characterisation of a protease from Escherichia coli involved in hydrogenase maturation. Eur J Biochem 227:545–550
Roseboom W, Blokesch M, Böck A, Albracht SP (2005) The biosynthetic routes of carbon monoxide and cyanide in the Ni-Fe active site of hydrogenases are different. FEBS Lett 579:469–472
Rubach JK, Brazzolotto X, Gaillard J, Fontecave M (2005) Biochemical characterization of the HydE and HydG iron-only hydrogenase maturation enzymes from Thermatoga maritima. FEBS Lett 579:5055–5060
Sargent F, Ballantine SP, Rugman PA, Palmer T, Boxer DH (1998) Reassignment of the gene encoding the Escherichia coli hydrogenase 2 small subunit: identification of a soluble precursor of the small subunit in a hypB mutant. Eur J Biochem 255:746–754
Sauter M, Böhm R, Böck A (1992) Mutational analysis of the operon (hyc) determining hydrogenase 3 formation in Escherichia coli. Mol Microbiol 6:1523–1532
Sawers RG, Boxer DH (1986) Purification and properties of membrane-bound hydrogenase isoenzyme 1 from anaerobically grown Escherichia coli K12. Eur J Biochem 156:265–275
Sawers RG, Ballantine SP, Boxer DH (1985) Differential expression of hydrogenase isoenzymes in Escherichia coli K-12: evidence for a third isoenzyme. J Bacteriol 164:1324–1331
Sawers RG, Blokesch M, Böck A (2004) Anaerobic formate and hydrogen metabolism. September (2004), posting date. Chapter 3.5.4. In: Curtiss III R (Editor in Chief), EcoSal–Escherichia coli and Salmonella: cellular and molecular biology. ASM Press, Washington, D.C. [Online] http://www.ecosal.org
Self WT, Hasona A, Shanmugam KT (2004) Expression and regulation of a silent operon, hyf, coding for hydrogenase 4 isoenzyme in Escherichia coli. J Bacteriol 186:580–587
Skibinski DAG, Golby P, Chang Y-S et al (2002) Regulation of the hydrogenase-4 operon of Escherichia coli by the σ54-dependent transcriptional activators FhlA and HyfR. J Bacteriol 184:6642–6653
Shima S, Lyon EJ, Sordel-Klippert M, Kauß M et al (2004) The cofactor of the iron-sulfur cluster free hydrogenase Hmd: structure of the light-inactivation product. Angew Chemie Int Ed 43:2547–2551
Stephenson M, Stickland LH (1931) Hydrogenase: a bacterial enzyme activating molecular hydrogen. I. The properties of the enzyme. Biochem J 25:205–214
Tard C, Liu XM, Ibrahim SK et al (2005) Synthesis of the H-cluster framework of iron-only hydrogenase. Nature 433:610–613
Tenhunen R, Marver HS, Schmid R (1969) Microsomal heme oxygenase: charaterization of the enzyme. J Biol Chem 244:6388–6294
Thauer RK (1990) Energy metabolism of methanogenic bacteria. Biochim Biophys Acta 1018:256–259
Theodoratou E, Paschos A, Magalon A, Fritsche E, Huber R, Böck A (2000) Nickel serves as substrate recognition motif for the endopeptidase involved in hydrogenase maturation. Eur J Biochem 267:1995–1999
Van der Spek TM, Arendsen AF, Happe RP et al (1996) Similarities in the architecture of the active sites of Ni-hydrogenases and Fe-hydrogenases detected by means of infrared spectroscopy. Eur J Biochem 237:629–634
Vignais PM, Colbeau A (2004) Molecular biology of microbial hydrogenases. Curr Issues Mol Biol 6:159–188
Volbeda A, Charon MH, Piras C, Hatchikian EC, Frey M, Fontecilla-Camps J (1995) Crystal structure of the nickel-iron hydrogenase from Desulfovibrio gigas. Nature 373:580–587
Volbeda A, Martin L, Cavazza C et al (2005) Structural differences between the ready and unready oxidized states of [NiFe] hydrogenases. J Biol Inorg Chem 10:239–249
Waugh R, Boxer DH (1986) Pleiotropic hydrogenase mutants of Escherichia coli K12: growth in the presence of nickel can restore hydrogenase activity. Biochimie 68:157–166
Zalkin H (1997) Formyltetrahydrofolate hydrolase from Escherichia coli. Meth Enzymol 281:214–218
Zhang JW, Butland G, Greenblatt JF, Emili A, Zamble DB (2005) A role for SlyD in the Escherichia coli hydrogenase biosynthetic pathway. J Biol Chem 280:4360–4366
Zinoni F, Birkmann A, Stadtman TC, Böck A (1986) Nucleotide sequence and expression of the selenocysteine-containing polypeptide of formate dehydrogenase (formate-hydrogen-lyase-linked) from Escherichia coli. Proc Natl Acad Sci USA 83:4650–4654
Zirngibl C, van Dongen W, Schwörer B et al (1992) H2-forming methylenetetrahydromethanopterin dehydrogenase, a novel type of hydrogenase without iron-sulfur clusters in methanogenic archaea. Eur J Biochem 208:511–520
Acknowledgements
We are indebted to August Böck and Rolf Thauer for their comments on the manuscript. The work described here has been supported by the Biotechnology and Biological Sciences Research Council, the Deutsche Forschungsgemeinschaft and the Max-Planck Society. Chris Pickett is thanked for useful discussions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Forzi, L., Sawers, R.G. Maturation of [NiFe]-hydrogenases in Escherichia coli . Biometals 20, 565–578 (2007). https://doi.org/10.1007/s10534-006-9048-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10534-006-9048-5