Abstract
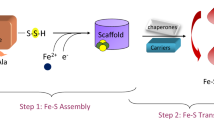
Iron–sulfur clusters are one of the most ubiquitous redox centers in biology. Ironically, iron-sulfur clusters are highly sensitive to reactive oxygen species. Disruption of iron-sulfur clusters will not only change the activity of proteins that host iron–sulfur clusters, the iron released from the disrupted iron–sulfur clusters will further promote the production of deleterious hydroxyl free radicals via the Fenton reaction. Here, we report that ferritin A (FtnA), a major iron-storage protein in Escherichia coli, is able to scavenge the iron released from the disrupted iron–sulfur clusters and alleviates the production of hydroxyl free radicals. Furthermore, we find that the iron stored in FtnA can be retrieved by an iron chaperon IscA for the re-assembly of the iron–sulfur cluster in a proposed scaffold IscU in the presence of the thioredoxin reductase system which emulates normal intracellular redox potential. The results suggest that E. coli FtnA may act as an iron buffer to sequester the iron released from the disrupted iron–sulfur clusters under oxidative stress conditions and to facilitate the re-assembly of the disrupted iron–sulfur clusters under normal physiological conditions.








Similar content being viewed by others
Abbreviations
- FtnA:
-
E. coli ferritin A
- IscA:
-
A proposed iron donor for the iron–sulfur cluster assembly
- IscS:
-
Cysteine desulfurase
- IscU:
-
Iron–sulfur cluster assembly scaffold protein
References
Abdul-Tehrani H, Hudson AJ, Chang YS, Timms AR, Hawkins C, Williams JM et al (1999) Ferritin mutants of Escherichia coli are iron deficient and growth impaired, and fur mutants are iron deficient. J Bacteriol 181:1415–1428
Agar JN, Krebs C, Frazzon J, Huynh BH, Dean DR, Johnson MK (2000) IscU as a scaffold for iron–sulfur cluster biosynthesis: sequential assembly of [2Fe–2S] and [4Fe–4S] clusters in IscU. Biochemistry 39:7856–7862. doi:10.1021/bi000931n
Andrews SC, Robinson AK, Rodriguez-Quinones F (2003) Bacterial iron homeostasis. FEMS Microbiol Rev 27:215–237. doi:10.1016/S0168-6445(03)00055-X
Aslund F, Beckwith J (1999) The thioredoxin superfamily: redundancy, specificity, and gray-area genomics. J Bacteriol 181:1375–1379
Beinert H, Holm RH, Munck E (1997) Iron–sulfur clusters: nature’s modular, multipurpose structures. Science 277:653–659. doi:10.1126/science.277.5326.653
Bou-Abdallah F, Woodhall MR, Velazquez-Campoy A, Andrews SC, Chasteen ND (2005) Thermodynamic analysis of ferrous ion binding to Escherichia coli ferritin EcFtnA. Biochemistry 44:13837–13846. doi:10.1021/bi0514212
Carrondo MA (2003) Ferritins, iron uptake and storage from the bacterioferritin viewpoint. EMBO J 22:1959–1968. doi:10.1093/emboj/cdg215
Chandramouli K, Johnson MK (2006) HscA and HscB stimulate [2Fe–2S] cluster transfer from IscU to apoferredoxin in an ATP-dependent reaction. Biochemistry 45:11087–11095. doi:10.1021/bi061237w
Cowart RE, Singleton FL, Hind JS (1993) A comparison of bathophenanthrolinedisulfonic acid and ferrozine as chelators of iron(II) in reduction reactions. Anal Biochem 211:151–155. doi:10.1006/abio.1993.1246
Cozzi A, Corsi B, Levi S, Santambrogio P, Albertini A, Arosio P (2000) Overexpression of wild type and mutated human ferritin H-chain in HeLa cells: in vivo role of ferritin ferroxidase activity. J Biol Chem 275:25122–25129. doi:10.1074/jbc.M003797200
Cupp-Vickery JR, Urbina H, Vickery LE (2003) Crystal structure of IscS, a cysteine desulfurase from Escherichia coli. J Mol Biol 330:1049–1059. doi:10.1016/S0022-2836(03)00690-9
De Domenico I, Vaughn MB, Li L, Bagley D, Musci G, Ward DM et al (2006) Ferroportin-mediated mobilization of ferritin iron precedes ferritin degradation by the proteasome. EMBO J 25:5396–5404. doi:10.1038/sj.emboj.7601409
Ding H, Clark RJ (2004) Characterization of iron binding in IscA, an ancient iron–sulphur cluster assembly protein. Biochem J 379:433–440. doi:10.1042/BJ20031702
Ding H, Clark RJ, Ding B (2004) IscA mediates iron delivery for assembly of iron–sulfur clusters in IscU under the limited accessible free iron conditions. J Biol Chem 279:37499–37504. doi:10.1074/jbc.M404533200
Ding B, Smith ES, Ding H (2005a) Mobilization of the iron centre in IscA for the iron–sulphur cluster assembly in IscU. Biochem J 389:797–802. doi:10.1042/BJ20050405
Ding H, Harrison K, Lu J (2005b) Thioredoxin reductase system mediates iron binding in IscA and iron delivery for the iron–sulfur cluster assembly in IscU. J Biol Chem 280:30432–30437. doi:10.1074/jbc.M504638200
Ding H, Yang J, Coleman LC, Yeung S (2007) Distinct iron binding property of two putative iron donors for the iron–sulfur cluster assembly: IscA and the bacterial frataxin ortholog cyay under physiological and oxidative stress conditions. J Biol Chem 282:7997–8004. doi:10.1074/jbc.M609665200
Djaman O, Outten FW, Imlay JA (2004) Repair of oxidized iron–sulfur clusters in Escherichia coli. J Biol Chem 279:44590–44599. doi:10.1074/jbc.M406487200
Epsztejn S, Glickstein H, Picard V, Slotki IN, Breuer W, Beaumont C et al (1999) H-ferritin subunit overexpression in erythroid cells reduces the oxidative stress response and induces multidrug resistance properties. Blood 94:3593–3603
Flint DH (1996) Escherichia coli contains a protein that is homologous in function and N-terminal sequence to the protein encoded by the nifS gene of Azotobacter vinelandii and that can participate in the synthesis of the Fe–S cluster of dihydroxy-acid dehydratase. J Biol Chem 271:16068–16074
Fontecave M (2006) Iron–sulfur clusters: ever-expanding roles. Nat Chem Biol 2:171–174. doi:10.1038/nchembio0406-171
Galatro A, Puntarulo S (2007) Mitochondrial ferritin in animals and plants. Front Biosci 12:1063–1071. doi:10.2741/2126
Halliwell B, Gutteridge JM, Aruoma OI (1987) The deoxyribose method: a simple “test-tube” assay for determination of rate constants for reactions of hydroxyl radicals. Anal Biochem 165:215–219. doi:10.1016/0003-2697(87)90222-3
Hudson AJ, Andrews SC, Hawkins C, Williams JM, Izuhara M, Meldrum FC et al (1993) Overproduction, purification and characterization of the Escherichia coli ferritin. Eur J Biochem 218:985–995. doi:10.1111/j.1432-1033.1993.tb18457.x
Jang S, Imlay JA (2007) Micromolar intracellular hydrogen peroxide disrupts metabolism by damaging iron–sulfur enzymes. J Biol Chem 282:929–937. doi:10.1074/jbc.M607646200
Johnson DC, Dean DR, Smith AD, Johnson MK (2005) Structure, function, and formation of biological iron–sulfur clusters. Annu Rev Biochem 74:247–281. doi:10.1146/annurev.biochem.74.082803.133518
Kato S, Mihara H, Kurihara T, Takahashi Y, Tokumoto U, Yoshimura T et al (2002) Cys-328 of IscS and Cys-63 of IscU are the sites of disulfide bridge formation in a covalently bound IscS/IscU complex: implications for the mechanism of iron–sulfur cluster assembly. Proc Natl Acad Sci USA 99:5948–5952. doi:10.1073/pnas.082123599
Keyer K, Imlay JA (1996) Superoxide accelerates DNA damage by elevating free-iron levels. Proc Natl Acad Sci USA 93:13635–13640. doi:10.1073/pnas.93.24.13635
Kidane TZ, Sauble E, Linder MC (2006) Release of iron from ferritin requires lysosomal activity. Am J Physiol Cell Physiol 291:C445–C455. doi:10.1152/ajpcell.00505.2005
Kiley PJ, Beinert H (2003) The role of Fe–S proteins in sensing and regulation in bacteria. Curr Opin Microbiol 6:181–185. doi:10.1016/S1369-5274(03)00039-0
Krebs C, Agar JN, Smith AD, Frazzon J, Dean DR, Huynh BH et al (2001) IscA, an alternate scaffold for Fe–S cluster biosynthesis. Biochemistry 40:14069–14080. doi:10.1021/bi015656z
Layer G, Gaddam SA, Ayala-Castro CN, Ollagnier-de-Choudens S, Lascoux D, Fontecave M et al (2007) SufE transfers sulfur from SufS to SufB for iron–sulfur cluster assembly. J Biol Chem 282:13342–13350. doi:10.1074/jbc.M608555200
Lill R, Muhlenhoff U (2006) Iron–sulfur protein biogenesis in eukaryotes: components and mechanisms. Annu Rev Cell Dev Biol 22:457–486. doi:10.1146/annurev.cellbio.22.010305.104538
Liu X, Theil EC (2005) Ferritins: dynamic management of biological iron and oxygen chemistry. Acc Chem Res 38:167–175. doi:10.1021/ar0302336
Liu X, Jin W, Theil EC (2003) Opening protein pores with chaotropes enhances Fe reduction and chelation of Fe from the ferritin biomineral. Proc Natl Acad Sci USA 100:3653–3658. doi:10.1073/pnas.0636928100
Liu XS, Patterson LD, Miller MJ, Theil EC (2007) Peptides selected for the protein nanocage pores change the rate of iron recovery from the ferritin mineral. J Biol Chem 282:31821–31825. doi:10.1074/jbc.C700153200
Loiseau L, Ollagnier-de-Choudens S, Nachin L, Fontecave M, Barras F (2003) Biogenesis of Fe–S cluster by the bacterial Suf system: SufS and SufE form a new type of cysteine desulfurase. J Biol Chem 278:38352–38359. doi:10.1074/jbc.M305953200
Lu J, Yang J, Tan G, Ding H (2008) Complementary roles of SufA and IscA in the biogenesis of iron–sulfur clusters in Escherichia coli. Biochem J 409:535–543. doi:10.1042/BJ20071166
Mulrooney SB (1997) Application of a single-plasmid vector for mutagenesis and high-level expression of thioredoxin reductase and its use to examine flavin cofactor incorporation. Protein Expr Purif 9:372–378. doi:10.1006/prep. 1996.0698
Ollagnier-de-Choudens S, Mattioli T, Takahashi Y, Fontecave M (2001) Iron–sulfur cluster assembly: characterization of IscA and evidence for a specific and functional complex with ferredoxin. J Biol Chem 276:22604–22607. doi:10.1074/jbc.M102902200
Outten FW, Wood MJ, Munoz FM, Storz G (2003) The SufE protein and the SufBCD complex enhance SufS cysteine desulfurase activity as part of a sulfur transfer pathway for Fe–S cluster assembly in Escherichia coli. J Biol Chem 278:45713–45719. doi:10.1074/jbc.M308004200
Outten FW, Djaman O, Storz G (2004) A suf operon requirement for Fe-S cluster assembly during iron starvation in Escherichia coli. Mol Microbiol 52:861–872. doi:10.1111/j.1365-2958.2004.04025.x
Radisky DC, Kaplan J (1998) Iron in cytosolic ferritin can be recycled through lysosomal degradation in human fibroblasts. Biochem J 336(Pt 1):201–205
Rouault TA, Tong WH (2005) Iron–sulphur cluster biogenesis and mitochondrial iron homeostasis. Nat Rev Mol Cell Biol 6:345–351. doi:10.1038/nrm1620
Sargent PJ, Farnaud S, Evans RW (2005) Structure/function overview of proteins involved in iron storage and transport. Curr Med Chem 12:2683–2693. doi:10.2174/092986705774462969
Sendra M, Ollagnier-de-Choudens S, Lascoux D, Sanakis Y, Fontecave M (2007) The SUF iron–sulfur cluster biosynthetic machinery: sulfur transfer from the SUFS-SUFE complex to SUFA. FEBS Lett 581:1362–1368. doi:10.1016/j.febslet.2007.02.058
Silberg JJ, Tapley TL, Hoff KG, Vickery LE (2004) Regulation of the HscA ATPase reaction cycle by the co-chaperone HscB and the iron–sulfur cluster assembly protein IscU. J Biol Chem 279:53924–53931. doi:10.1074/jbc.M410117200
Smith AD, Agar JN, Johnson KA, Frazzon J, Amster IJ, Dean DR et al (2001) Sulfur transfer from IscS to IscU: the first step in iron–sulfur cluster biosynthesis. J Am Chem Soc 123:11103–11104. doi:10.1021/ja016757n
Smith AD, Frazzon J, Dean DR, Johnson MK (2005) Role of conserved cysteines in mediating sulfur transfer from IscS to IscU. FEBS Lett 579:5236–5240. doi:10.1016/j.febslet.2005.08.046
Takahashi Y, Tokumoto U (2002) A third bacterial system for the assembly of iron–sulfur clusters with homologs in archaea and plastids. J Biol Chem 277:28380–28383. doi:10.1074/jbc.C200365200
Tapley TL, Vickery LE (2004) Preferential substrate binding orientation by the molecular chaperone HscA. J Biol Chem 279:28435–28442. doi:10.1074/jbc.M400803200
Unciuleac MC, Chandramouli K, Naik S, Mayer S, Huynh BH, Johnson MK et al (2007) In vitro activation of apo-aconitase using a [4Fe–4S] cluster-loaded form of the IscU [Fe–S] cluster scaffolding protein. Biochemistry 46:6812–6821. doi:10.1021/bi6026665
Urbina HD, Silberg JJ, Hoff KG, Vickery LE (2001) Transfer of sulfur from IscS to IscU during Fe/S cluster assembly. J Biol Chem 276:44521–44526. doi:10.1074/jbc.M106907200
Veine DM, Mulrooney SB, Wang PF, Williams CH Jr (1998) Formation and properties of mixed disulfides between thioredoxin reductase from Escherichia coli and thioredoxin: evidence that cysteine-138 functions to initiate dithiol-disulfide interchange and to accept the reducing equivalent from reduced flavin. Protein Sci 7:1441–1450
Velayudhan J, Castor M, Richardson A, Main-Hester KL, Fang FC (2007) The role of ferritins in the physiology of Salmonella enterica sv. Typhimurium: a unique role for ferritin B in iron–sulphur cluster repair and virulence. Mol Microbiol 63:1495–1507. doi:10.1111/j.1365-2958.2007.05600.x
Wollenberg M, Berndt C, Bill E, Schwenn JD, Seidler A (2003) A dimer of the FeS cluster biosynthesis protein IscA from cyanobacteria binds a [2Fe–2S] cluster between two protomers and transfers it to [2Fe–2S] and [4Fe–4S] apo proteins. Eur J Biochem 270:1662–1671. doi:10.1046/j.1432-1033.2003.03522.x
Wu SP, Wu G, Surerus KK, Cowan JA (2002) Iron–sulfur cluster biosynthesis, kinetic analysis of [2Fe–2S] cluster transfer from holo ISU to apo Fd: role of redox chemistry and a conserved aspartate. Biochemistry 41:8876–8885. doi:10.1021/bi0256781
Yang W, Rogers PA, Ding H (2002) Repair of nitric oxide modified ferredoxin [2Fe–2S] cluster by cysteine desulfurase (IscS). J Biol Chem 277:12868–12873. doi:10.1074/jbc.M109485200
Yang J, Bitoun JP, Ding H (2006) Interplay of IscA and IscU in biogenesis of iron–sulfur clusters. J Biol Chem 281:27956–27963. doi:10.1074/jbc.M601356200
Zhao G, Arosio P, Chasteen ND (2006) Iron(II) and hydrogen peroxide detoxification by human H-chain ferritin. An EPR spin-trapping study. Biochemistry 45:3429–3436. doi:10.1021/bi052443r
Zheng L, Cash VL, Flint DH, Dean DR (1998) Assembly of iron–sulfur clusters. Identification of an iscSUA-hscBA-fdx gene cluster from Azotobacter vinelandii. J Biol Chem 273:13264–13272. doi:10.1074/jbc.273.21.13264
Acknowledgement
This work was supported in part by the National Sciences Foundation grant (MCB-0416537) and the National Institutes of Health grant (CA107494) to H.D.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bitoun, J.P., Wu, G. & Ding, H. Escherichia coli FtnA acts as an iron buffer for re-assembly of iron–sulfur clusters in response to hydrogen peroxide stress. Biometals 21, 693–703 (2008). https://doi.org/10.1007/s10534-008-9154-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10534-008-9154-7