Abstract
Purpose
To study the prognostic significance of elevated cytokeratin 19 (CK19) mRNA levels in the bone marrow (BM) of operable breast cancer patients.
Patients and Methods
From 1998 to 2000, BM was collected from 195 consecutive breast cancer patients immediately prior to surgery and from 34 healthy volunteers. The patients received surgical and adjuvant treatment according to national guidelines at the time. We analyzed the level of CK19 mRNA in the BM samples from patients and normal controls using a real-time RT-PCR assay. The associations with known prognostic factors and the impact of pathological CK19 mRNA levels on patients’ prognosis were investigated.
Results
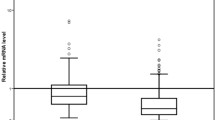
Using the 99 percentile of the normal control group as a cut-off, 24 (12%) of the 195 patients and 1 (3%) of the 34 volunteers were diagnosed as CK19 mRNA positive. There was no correlation between CK19 BM status and the clinicopathological factors tested. During a median follow-up of 72 months, 7 (29%) of the 24 CK19 mRNA BM positive patients experienced systemic relapse compared to 20 (12%) of the 171 in the CK19 mRNA negative group. The patients with CK19 mRNA-positive BM had significantly shorter systemic recurrence-free survival (P = 0.01) and overall recurrence-free survival (P = 0.005). Multivariate Cox regression showed CK19 mRNA BM status to be an independent predictor of relapse.
Conclusion
Detection of CK19 mRNA in the BM of breast cancer patients by real-time RT-PCR is an independent predictor of relapse-free survival in operable breast cancer patients.


Similar content being viewed by others
References
Heimann R, Hellman S (2000) Clinical progression of breast cancer malignant behavior: what to expect and when to expect it. J Clin Oncol 18(3):591–599
Slade MJ, Singh A, Smith BM, Tripuraneni G, Hall E, Peckitt C et al (2005) Persistence of bone marrow micrometastases in patients receiving adjuvant therapy for breast cancer: results at 4 years. Int J Cancer 114(1):94–100
Harbeck N, Untch M, Pache L, Eiermann W (1994) Tumour cell detection in the bone marrow of breast cancer patients at primary therapy: results of a 3-year median follow-up. Br J Cancer 69(3):566–571
Diel IJ, Kaufmann M, Costa SD, Holle R, von Minckwitz G, Solomayer EF et al (1996) Micrometastatic breast cancer cells in bone marrow at primary surgery: prognostic value in comparison with nodal status. J Natl Cancer Inst 88(22):1652–1658
Mansi JL, Gogas H, Bliss JM, Gazet JC, Berger U, Coombes RC (1999) Outcome of primary-breast-cancer patients with micrometastases: a long-term follow-up study. Lancet 354(9174):197–202
Braun S, Pantel K, Muller P, Janni W, Hepp F, Kentenich CR et al (2000) Cytokeratin-positive cells in the bone marrow and survival of patients with stage I, II, or III breast cancer. N Engl J Med 342(8):525–533
Solomayer EF, Diel IJ, Salanti G, Hahn M, Gollan C, Schutz F et al (2001) Time independence of the prognostic impact of tumor cell detection in the bone marrow of primary breast cancer patients. Clin Cancer Res 7(12):4102–4108
Gebauer G, Fehm T, Merkle E, Beck EP, Lang N, Jager W (2001) Epithelial cells in bone marrow of breast cancer patients at time of primary surgery: clinical outcome during long-term follow-up. J Clin Oncol 19(16):3669–3674
Wiedswang G, Borgen E, Karesen R, Kvalheim G, Nesland JM, Qvist H et al (2003) Detection of isolated tumor cells in bone marrow is an independent prognostic factor in breast cancer. J Clin Oncol 21(18):3469–3478
Pierga JY, Bonneton C, Vincent-Salomon A, de Cremoux P, Nos C, Blin N et al (2004) Clinical significance of immunocytochemical detection of tumor cells using digital microscopy in peripheral blood and bone marrow of breast cancer patients. Clin Cancer Res 10(4):1392–1400
Funke I, Schraut W (1998) Meta-analyses of studies on bone marrow micrometastases: an independent prognostic impact remains to be substantiated. J Clin Oncol 16(2):557–566
Braun S, Vogl FD, Naume B, Janni W, Osborne MP, Coombes RC et al (2005) A pooled analysis of bone marrow micrometastasis in breast cancer. N Engl J Med 353(8):793–802
Schoenfeld A, Kruger KH, Gomm J, Sinnett HD, Gazet JC, Sacks N et al (1997) The detection of micrometastases in the peripheral blood and bone marrow of patients with breast cancer using immunohistochemistry and reverse transcriptase polymerase chain reaction for keratin 19. Eur J Cancer 33(6):854–861
Ballestrero A, Coviello DA, Garuti A, Nencioni A, Fama A, Rocco I et al (2001) Reverse-transcriptase polymerase chain reaction of the maspin gene in the detection of bone marrow breast carcinoma cell contamination. Cancer 92(8):2030–2035
Fields KK, Elfenbein GJ, Trudeau WL, Perkins JB, Janssen WE, Moscinski LC (1996) Clinical significance of bone marrow metastases as detected using the polymerase chain reaction in patients with breast cancer undergoing high-dose chemotherapy and autologous bone marrow transplantation. J Clin Oncol 14(6):1868–1876
Slade MJ, Smith BM, Sinnett HD, Cross NC, Coombes RC (1999) Quantitative polymerase chain reaction for the detection of micrometastases in patients with breast cancer. J Clin Oncol 17(3):870–879
Stathopoulou A, Vlachonikolis I, Mavroudis D, Perraki M, Kouroussis Ch, Apostolaki S et al (2002) Molecular detection of cytokeratin-19-positive cells in the peripheral blood of patients with operable breast cancer: evaluation of their prognostic significance. J Clin Oncol 20(16):3404–3412
Benoy IH, Elst H, Van der Auwera I, Van Laere S, van Dam P, Van Marck E et al (2004) Real-time RT-PCR correlates with immunocytochemistry for the detection of disseminated epithelial cells in bone marrow aspirates of patients with breast cancer. Br J Cancer 91(10):1813–1820
Benoy IH, Elst H, Philips M, Wuyts H, Van Dam P, Scharpe S et al (2006) Real-time RT-PCR detection of disseminated tumour cells in bone marrow has superior prognostic significance in comparison with circulating tumour cells in patients with breast cancer. Br J Cancer 94(5):672–680
Benoy IH, Elst H, Philips M, Wuyts H, Van Dam P, Scharpe S et al (2006) Prognostic significance of disseminated tumor cells as detected by quantitative real-time reverse-transcriptase polymerase chain reaction in patients with breast cancer. Clin Breast Cancer 7(2):146–152
Shammas FV, Van Eekelen JA, Wee L, Heikkila R, Osland A (1999) Sensitive and quantitative one-step polymerase chain reaction using capillary electrophoresis and fluorescence detection for measuring cytokeratin 19 expression. Scand J Clin Lab Invest 59(8):635–642
Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A et al (2002) Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3(7):research0034.1–0034.11
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25(4):402–408
Nordgard O, Kvaloy JT, Farmen RK, Heikkila R (2006) Error propagation in relative real-time reverse transcription polymerase chain reaction quantification models: the balance between accuracy and precision. Anal Biochem 356(2):182–193
Pfaffl MW, Horgan GW, Dempfle L (2002) Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res 30(9):e36
Grambsch P, Therneau T (1994) Proportional hazards tests and diagnostics based on weighted residuals. Biometrika 81:515–526
Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, Miller MC et al (2004) Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med 351(8):781–791
Cristofanilli M, Hayes DF, Budd GT, Ellis MJ, Stopeck A, Reuben JM et al (2005) Circulating tumor cells: a novel prognostic factor for newly diagnosed metastatic breast cancer. J Clin Oncol 23(7):1420–1430
Wiedswang G, Borgen E, Schirmer C, Karesen R, Kvalheim G, Nesland JM et al (2006) Comparison of the clinical significance of occult tumor cells in blood and bone marrow in breast cancer. Int J Cancer 118(8):2013–2019
Xenidis N, Perraki M, Kafousi M, Apostolaki S, Bolonaki I, Stathopoulou A et al (2006) Predictive and prognostic value of peripheral blood cytokeratin-19 mRNA-positive cells detected by real-time polymerase chain reaction in node-negative breast cancer patients. J Clin Oncol 24(23):3756–3762
Schlimok G, Funke I, Holzmann B, Gottlinger G, Schmidt G, Hauser H et al (1987) Micrometastatic cancer cells in bone marrow: in vitro detection with anti-cytokeratin and in vivo labeling with anti-17-1A monoclonal antibodies. Proc Natl Acad Sci U S A 84(23):8672–8676
Abd El-Rehim DM, Pinder SE, Paish CE, Bell J, Blamey RW, Robertson JF et al (2004) Expression of luminal and basal cytokeratins in human breast carcinoma. J Pathol 203(2):661–671
Woelfle U, Cloos J, Sauter G, Riethdorf L, Janicke F, van Diest P et al (2003) Molecular signature associated with bone marrow micrometastasis in human breast cancer. Cancer Res 63(18):5679–5684
Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H et al (2001) Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A 98(19):10869–10874
Chambers AF, Naumov GN, Vantyghem SA, Tuck AB (2000) Molecular biology of breast cancer metastasis. Clinical implications of experimental studies on metastatic inefficiency. Breast Cancer Res 2(6):400–407
Mehes G, Witt A, Kubista E, Ambros PF (2001) Circulating breast cancer cells are frequently apoptotic. Am J Pathol 159(1):17–20
Mehlen P, Puisieux A (2006) Metastasis: a question of life or death. Nat Rev Cancer 6(6):449–458
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by the Norwegian Cancer Society.
Rights and permissions
About this article
Cite this article
Farmen, R.K., Nordgård, O., Gilje, B. et al. Bone marrow cytokeratin 19 mRNA level is an independent predictor of relapse-free survival in operable breast cancer patients. Breast Cancer Res Treat 108, 251–258 (2008). https://doi.org/10.1007/s10549-007-9592-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-007-9592-x