Abstract
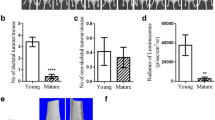
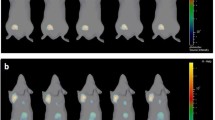
The development of novel diagnostic agents for the detection of breast cancer microcalcifications requires a reliable animal model. Based on previous work from our group, we hypothesized that a single systemic injection of recombinant bone morphogenetic protein-2 (rBMP-2) could be used to create such a model. The cDNA encoding mature human BMP-2 was expressed in BL21(DE3) bacteria, purified to homogeneity, and refolded as a dimer. Bioactivity was confirmed using a C2C12 alkaline phosphatase assay. rBMP-2 was radiolabeled with 99mTc, and its biodistribution and clearance were quantified after both intravenous (IV) and intraperitoneal (IP) injection. Fischer 344 rats bearing syngeneic R3230 breast tumors received a single intraperitoneal injection of rBMP-2 at a specified dose. Tumor microcalcification was quantified over time using micro–single photon emission computed tomography (SPECT) and microcomputed tomography (CT). rBMP-2 could be expressed in E. coli at high levels, isolated at >95% purity, and refolded to a bioactive dimer. Beta-phase half-life was 30.5 min after IV administration and 47.6 min after IP administration. Renal excretion was the primary mode of clearance. A single IP injection of ≥50 μg rBMP-2 when tumors were not yet palpable resulted in dose-dependent microcalcification in 8 of 8 R3230 tumors. No calcification was found in control tumors or in normal tissues and organs of animals injected with rBMP-2. Tumor calcification increased progressively between weeks 2 and 4 post-rBMP-2 injection. A single IP injection of rBMP-2 in rats bearing a syngeneic breast cancer will produce dose-dependent and time-dependent microcalcifications. This animal model lays the foundation for the development of novel diagnostic radiotracers for breast cancer.




Similar content being viewed by others
Abbreviations
- %ID:
-
Percent injected dose
- %ID/g:
-
Percent injected dose per gram of tissue
- aa:
-
Amino acids
- ALP:
-
Alkaline phosphatase
- BMP-2:
-
Bone morphogenetic protein-2
- CT:
-
Computed tomography
- CV:
-
Column volumes
- DCE:
-
Dynamic contrast-enhanced
- DMEM:
-
Dulbecco’s modified Eagle’s medium
- DMSO:
-
Dimethyl sulfoxide
- FBS:
-
Fetal bovine serum
- Gnd-HCl:
-
Guanidinium hydrochloride
- HA:
-
Hydroxyapatite
- H&E:
-
Hematoxylin and eosin
- IP:
-
Intraperitoneal
- IPTG:
-
Isopropyl β-d-1-thiogalactopyranoside
- IV:
-
Intravenous
- MDP:
-
Methylene diphosphonate
- MIP:
-
Maximal intensity projection
- MRI:
-
Magnetic resonance imaging
- NHS:
-
N-hydroxysuccinimide
- PBS:
-
Phosphate-buffered saline
- PCR:
-
Polymerase chain reaction
- PEM:
-
Positron emission mammography
- PET:
-
Positron emission tomography
- PPV:
-
Positive predictive value
- rBMP-2:
-
Recombinant bone morphogenetic protein-2
- SPECT:
-
Single-photon emission computed tomography
References
Feig SA, Sickles EA, Evans WP, Linver MN (2004) Re: changes in breast cancer detection and mammography recall rates after the introduction of a computer-aided detection system. J Natl Cancer Inst 96:1260–1261 author reply 1261
Morgan MP, Cooke MM, McCarthy GM (2005) Microcalcifications associated with breast cancer: an epiphenomenon or biologically significant feature of selected tumors? J Mammary Gland Biol Neoplasia 10:181–187
Stomper PC, Geradts J, Edge SB, Levine EG (2003) Mammographic predictors of the presence and size of invasive carcinomas associated with malignant microcalcification lesions without a mass. AJR Am J Roentgenol 181:1679–1684
Liberman L (2004) Breast cancer screening with MRI-what are the data for patients at high risk? N Engl J Med 351:497–500
Berg WA, Weinberg IN, Narayanan D, Lobrano ME, Ross E, Amodei L, Tafra L, Adler LP, Uddo J, Stein W 3rd et al (2006) High-resolution fluorodeoxyglucose positron emission tomography with compression (“positron emission mammography”) is highly accurate in depicting primary breast cancer. Breast J 12:309–323
Raylman RR, Majewski S, Smith MF, Proffitt J, Hammond W, Srinivasan A, McKisson J, Popov V, Weisenberger A, Judy CO et al (2008) The positron emission mammography/tomography breast imaging and biopsy system (PEM/PET): design, construction and phantom-based measurements. Phys Med Biol 53:637–653
Wu X, Xiao H (2009) Progress in the detection of human genome structural variations. Sci China C Life Sci 52:560–567
Ferranti C, Coopmans de Yoldi G, Biganzoli E, Bergonzi S, Mariani L, Scaperrotta G, Marchesini M (2000) Relationships between age, mammographic features and pathological tumour characteristics in non-palpable breast cancer. Br J Radiol 73:698–705
Evans AJ, Kutt E, Record C, Waller M, Bobrow L, Moss S (2007) Radiological and pathological findings of interval cancers in a multi-centre, randomized, controlled trial of mammographic screening in women from age 40–41 years. Clin Radiol 62:348–352
Bhushan KR, Misra P, Liu F, Mathur S, Lenkinski RE, Frangioni JV (2008) Detection of breast cancer microcalcifications using a dual-modality SPECT/NIR fluorescent probe. J Am Chem Soc 130:17648–17649
Bhushan KR, Tanaka E, Frangioni JV (2007) Synthesis of conjugatable bisphosphonates for molecular imaging of large animals. Angew Chem Int Ed Engl 46:7969–7971
Lenkinski RE, Ahmed M, Zaheer A, Frangioni JV, Goldberg SN (2003) Near-infrared fluorescence imaging of microcalcification in an animal model of breast cancer. Acad Radiol 10:1159–1164
Zaheer A, Lenkinski RE, Mahmood A, Jones AG, Cantley LC, Frangioni JV (2001) In vivo near-infrared fluorescence imaging of osteoblastic activity. Nat Biotechnol 19:1148–1154
Haka AS, Shafer-Peltier KE, Fitzmaurice M, Crowe J, Dasari RR, Feld MS (2002) Identifying microcalcifications in benign and malignant breast lesions by probing differences in their chemical composition using Raman spectroscopy. Cancer Res 62:5375–5380
Liu F, Bloch N, Bhushan KR, De Grand AM, Tanaka E, Solazzo S, Mertyna PM, Goldberg N, Frangioni JV, Lenkinski RE (2008) Humoral bone morphogenetic protein 2 is sufficient for inducing breast cancer microcalcification. Mol Imaging 7:175–186
Wozney JM, Rosen V, Celeste AJ, Mitsock LM, Whitters MJ, Kriz RW, Hewick RM, Wang EA (1988) Novel regulators of bone formation: molecular clones and activities. Science 242:1528–1534
Vallejo LF, Brokelmann M, Marten S, Trappe S, Cabrera-Crespo J, Hoffmann A, Gross G, Weich HA, Rinas U (2002) Renaturation and purification of bone morphogenetic protein-2 produced as inclusion bodies in high-cell-density cultures of recombinant Escherichia coli. J Biotechnol 94:185–194
Katagiri T, Yamaguchi A, Komaki M, Abe E, Takahashi N, Ikeda T, Rosen V, Wozney JM, Fujisawa-Sehara A, Suda T (1994) Bone morphogenetic protein-2 converts the differentiation pathway of C2C12 myoblasts into the osteoblast lineage. J Cell Biol 127:1755–1766
Misra P, Humblet V, Pannier N, Maison W, Frangioni JV (2007) Production of multimeric prostate-specific membrane antigen small-molecule radiotracers using a solid-phase 99mTc preloading strategy. J Nucl Med 48:1379–1389
Thompson SW, Hunt RD (1996) Selected histochemical and histopathological methods. Charles C. Thomas, Springfield
Zaheer A, Murshed M, De Grand AM, Morgan TG, Karsenty G, Frangioni JV (2006) Optical imaging of hydroxyapatite in the calcified vasculature of transgenic animals. Arterioscler Thromb Vasc Biol 26:1132–1136
Knaus P, Sebald W (2001) Cooperativity of binding epitopes and receptor chains in the BMP/TGFbeta superfamily. Biol Chem 382:1189–1195
Long S, Truong L, Bennett K, Phillips A, Wong-Staal F, Ma H (2006) Expression, purification, and renaturation of bone morphogenetic protein-2 from Escherichia coli. Protein Expr Purif 46:374–378
Steinert S, Kroll TC, Taubert I, Pusch L, Hortschansky P, Hoffken K, Wolfl S, Clement JH (2008) Differential expression of cancer-related genes by single and permanent exposure to bone morphogenetic protein 2. J Cancer Res Clin Oncol 134:1237–1245
Alarmo EL, Kuukasjarvi T, Karhu R, Kallioniemi A (2007) A comprehensive expression survey of bone morphogenetic proteins in breast cancer highlights the importance of BMP4 and BMP7. Breast Cancer Res Treat 103:239–246
Dimar JR 2nd, Glassman SD, Burkus JK, Pryor PW, Hardacker JW, Carreon LY (2009) Clinical and radiographic analysis of an optimized rhBMP-2 formulation as an autograft replacement in posterolateral lumbar spine arthrodesis. J Bone Joint Surg Am 91:1377–1386
Clement JH, Raida M, Sanger J, Bicknell R, Liu J, Naumann A, Geyer A, Waldau A, Hortschansky P, Schmidt A et al (2005) Bone morphogenetic protein 2 (BMP-2) induces in vitro invasion and in vivo hormone independent growth of breast carcinoma cells. Int J Oncol 27:401–407
Katsuno Y, Hanyu A, Kanda H, Ishikawa Y, Akiyama F, Iwase T, Ogata E, Ehata S, Miyazono K, Imamura T (2008) Bone morphogenetic protein signaling enhances invasion and bone metastasis of breast cancer cells through Smad pathway. Oncogene 27:6322–6333
Langenfeld EM, Calvano SE, Abou-Nukta F, Lowry SF, Amenta P, Langenfeld J (2003) The mature bone morphogenetic protein-2 is aberrantly expressed in non-small cell lung carcinomas and stimulates tumor growth of A549 cells. Carcinogenesis 24:1445–1454
Langenfeld EM, Langenfeld J (2004) Bone morphogenetic protein-2 stimulates angiogenesis in developing tumors. Mol Cancer Res 2:141–149
Scheufler C, Sebald W, Hulsmeyer M (1999) Crystal structure of human bone morphogenetic protein-2 at 2.7 A resolution. J Mol Biol 287:103–115
Acknowledgments
We thank Barbara L. Clough and Lorissa A. Moffitt for editing. This work was funded by NIH grant R01-CA-115296, NCRR shared instrumentation grant S10-RR-023010, and grants from the Lewis Family Fund and Ellison Foundation.
Competing interests:
Fangbing Liu: None.
Preeti Misra: None.
Elaine Lunsford: None
Joanne T. Vannah: None
Yuxia Liu: None
Robert E. Lenkinski: None
John V. Frangioni: None
Author Contributions
Fangbing Liu: Preparation of all reagents. Conducted all experiments. Wrote first draft of manuscript. Edited final draft of manuscript.
Preeti Misra: 99mTc-radiolabeling and purification of BMP-2. In vivo biodistribution and clearance experiments. Reviewed final draft of manuscript.
Elaine Lunsford: Micro-CT and micro-SPECT experiments and quantitative analysis of data. Reviewed final draft of manuscript.
Joanne T. Vannah: Micro-CT and micro-SPECT experiments and quantitative analysis of data. Reviewed final draft of manuscript.
Yuxia Liu: Micro-CT and micro-SPECT experiments and quantitative analysis of data. Reviewed final draft of manuscript.
Robert E. Lenkinski: Design of study. Review and interpretation of all primary data. Edited final draft of manuscript.
John V. Frangioni: Design of study. Review and interpretation of all primary data. Wrote final draft of manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, F., Misra, P., Lunsford, E.P. et al. A dose- and time-controllable syngeneic animal model of breast cancer microcalcification. Breast Cancer Res Treat 122, 87–94 (2010). https://doi.org/10.1007/s10549-009-0535-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-009-0535-6