Abstract
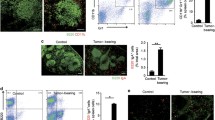
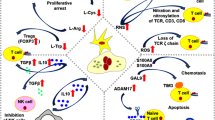
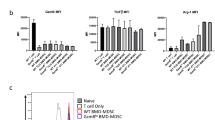
Recent reports have shown the involvement of tumor burden as well as GM-CSF in supporting myeloid-derived suppressor cells (MDSC). However, it is not known what progenitor cells may differentiate into MDSC in the presence of GM-CSF, and whether FVBN202 transgenic mouse model of spontaneous breast carcinoma may exhibit distinct subset distribution of CD11b+Gr1+ cells. In addition, it is not known why CD11b+Gr1+ cells derived from tumor-free and tumor-bearing animals exhibit different functions. In this study, we determined that GM-CSF was one of the tumor-derived soluble factors that induced differentiation of CD11b-Gr1- progenitor cells from within monocytic/granulocytic bone marrow cells into CD11b+Gr1+ cells. We also showed that CD11b+Gr1+ cells in FVBN202 mice consisted of CD11b+Ly6G-Ly6C+ suppressive and CD11b+Ly6G+Ly6C+ non-suppressive subsets. Previously reported variations between tumor-free and tumor-bearing animals in the function of their CD11b+Gr1+ cells were found to be due to the variations in the proportion of these two subsets. Therefore, increasing ratios of CD11b+Gr1+ cells derived from tumor-free animals revealed their suppressive activity on T cells, in vitro. Importantly, GM-CSF supported the generation of CD11b+Ly6G-Ly6C+ suppressor subsets that inhibited proliferation as well as anti-tumor function of neu-specific T cells. These findings suggest revisiting the use of GM-CSF for the expansion of dendritic cells, ex vivo, for cell-based immunotherapy or as an adjuvant for vaccines for patients with cancer in whom MDSC play a major role in the suppression of anti-tumor immune responses.





Similar content being viewed by others
References
Gallina G, Dolcetti L, Serafini P et al (2006) Tumors induce a subset of inflammatory monocytes with immunosuppressive activity on CD8+ T cells. J Clin Invest 116:2777–2790
Youn JI, Nagaraj S, Collazo M, Gabrilovich DI (2008) Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J Immunol 181:5791–5802
Habibi M, Kmieciak M, Graham L, Morales JK, Bear HD, Manjili MH (2009) Radiofrequency thermal ablation of breast tumors combined with intralesional administration of IL-7 and IL-15 augments anti-tumor immune responses and inhibits tumor development and metastasis. Breast Cancer Res Treat 114:423–431
Rodriguez PC, Hernandez CP, Quiceno D et al (2005) Arginase I in myeloid suppressor cells is induced by COX-2 in lung carcinoma. J Exp Med 202:931–939
Ochoa AC, Zea AH, Hernandez C, Rodriguez PC (2007) Arginase, prostaglandins, and myeloid-derived suppressor cells in renal cell carcinoma. Clin Cancer Res 13:721s–726s
Zea AH, Rodriguez PC, Atkins MB et al (2005) Arginase-producing myeloid suppressor cells in renal cell carcinoma patients: a mechanism of tumor evasion. Cancer Res 65:3044–3048
Valenti R, Huber V, Filipazzi P et al (2006) Human tumor-released microvesicles promote the differentiation of myeloid cells with transforming growth factor-beta-mediated suppressive activity on T lymphocytes. Cancer Res 66:9290–9298
Young MR, Lathers DM (1999) Myeloid progenitor cells mediate immune suppression in patients with head and neck cancers. Int J Immunopharmacol 21:241–252
Diaz-Montero CM, Salem ML, Nishimura MI, Garrett-Mayer E, Cole DJ, Montero AJ (2009) Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer Immunol Immunother 58:49–59
Ezernitchi AV, Vaknin I, Cohen-Daniel L et al (2006) TCR zeta down-regulation under chronic inflammation is mediated by myeloid suppressor cells differentially distributed between various lymphatic organs. J Immunol 177:4763–4772
Gruber IV, El Yousfi S, Durr-Storzer S, Wallwiener D, Solomayer EF, Fehm T (2008) Down-regulation of CD28, TCR-zeta (zeta) and up-regulation of FAS in peripheral cytotoxic T-cells of primary breast cancer patients. Anticancer Res 28:779–784
Dworacki G, Meidenbauer N, Kuss I, Hoffmann TK, Gooding W, Lotze M, Whiteside TL (2001) Decreased zeta chain expression and apoptosis in CD3+ peripheral blood T lymphocytes of patients with melanoma. Clin Cancer Res 7:947s–957s
Takahashi A, Kono K, Amemiya H, Iizuka H, Fujii H, Matsumoto Y (2001) Elevated caspase-3 activity in peripheral blood T cells coexists with increased degree of T-cell apoptosis and down-regulation of TCR zeta molecules in patients with gastric cancer. Clin Cancer Res 7:74–80
Pan PY, Wang GX, Yin B et al (2008) Reversion of immune tolerance in advanced malignancy: modulation of myeloid-derived suppressor cell development by blockade of stem-cell factor function. Blood 111:219–228
Melani C, Chiodoni C, Forni G, Colombo MP (2003) Myeloid cell expansion elicited by the progression of spontaneous mammary carcinomas in c-erbB-2 transgenic BALB/c mice suppresses immune reactivity. Blood 102:2138–2145
Garrity T, Pandit R, Wright MA, Benefield J, Keni S, Young MR (1997) Increased presence of CD34+ cells in the peripheral blood of head and neck cancer patients and their differentiation into dendritic cells. Int J Cancer 73:663–669
Parmiani G, Castelli C, Pilla L, Santinami M, Colombo MP, Rivoltini L (2007) Opposite immune functions of GM-CSF administered as vaccine adjuvant in cancer patients. Ann Oncol 18:226–232
Rosenberg SA, Yang JC, Schwartzentruber DJ et al (1999) Impact of cytokine administration on the generation of antitumor reactivity in patients with metastatic melanoma receiving a peptide vaccine. J Immunol 163:1690–1695
Filipazzi P, Valenti R, Huber V et al (2007) Identification of a new subset of myeloid suppressor cells in peripheral blood of melanoma patients with modulation by a granulocyte-macrophage colony-stimulation factor-based antitumor vaccine. J Clin Oncol 25:2546–2553
Serafini P, Carbley R, Noonan KA, Tan G, Bronte V, Borrello I (2004) High-dose granulocyte-macrophage colony-stimulating factor-producing vaccines impair the immune response through the recruitment of myeloid suppressor cells. Cancer Res 64:6337–6343
Rössner S, Voigtländer C, Wiethe C, Hänig J, Seifarth C, Lutz MB (2005) Myeloid dendritic cell precursors generated from bone marrow suppress T cell responses via cell contact and nitric oxide production in vitro. Eur J Immunol 35(12):3533–3544
Movahedi K, Guilliams M, Van den Bossche J et al (2008) Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T cell-suppressive activity. Blood 111:4233–4244
Sawanobori Y, Ueha S, Kurachi M et al (2008) Chemokine-mediated rapid turnover of myeloid-derived suppressor cells in tumor-bearing mice. Blood 111:5457–5466
Guy CT, Webster MA, Schaller M, Parsons TJ, Cardiff RD, Muller WJ (1992) Expression of the neu protooncogene in the mammary epithelium of transgenic mice induces metastatic disease. Proc Natl Acad Sci USA 89:10578–10582
Kmieciak M, Morales JK, Morales J, Bolesta E, Grimes M, Manjili MH (2008) Danger signals and nonself entity of tumor antigen are both required for eliciting effective immune responses against HER-2/neu positive mammary carcinoma: implications for vaccine design. Cancer Immunol Immunother 57:1391–1398
Kmieciak M, Knutson KL, Dumur CI, Manjili MH (2007) HER-2/neu antigen loss and relapse of mammary carcinoma are actively induced by T cell-mediated anti-tumor immune responses. Eur J Immunol 37:675–685
Morales JK, Kmieciak M, Graham L, Feldmesser M, Bear HD, Manjili MH (2009) Adoptive transfer of HER2/neu-specific T cells expanded with alternating gamma chain cytokines mediate tumor regression when combined with the depletion of myeloid-derived suppressor cells. Cancer Immunol Immunother 58(6):941–953
Dugast AS, Haudebourg T, Coulon F et al (2008) Myeloid-derived suppressor cells accumulate in kidney allograft tolerance and specifically suppress effector T cell expansion. J Immunol 180(12):7898–7906
Marigo I, Dolcetti L, Serafini P, Zanovello P, Bronte V (2008) Tumor-induced tolerance and immune suppression by myeloid derived suppressor cells. Immunol Rev 222:162–179
Bronte V, Chappell DB, Apolloni E et al (1999) Unopposed production of granulocyte-macrophage colony-stimulating factor by tumors inhibits CD8+ T cell responses by dysregulating antigen-presenting cell maturation. J Immunol 162:5728–5737
Daud AI, Mirza N, Lenox B et al (2008) Phenotypic and functional analysis of dendritic cells and clinical outcome in patients with high-risk melanoma treated with adjuvant granulocyte macrophage colony-stimulating factor. J Clin Oncol 26:3235–3241
Nefedova Y, Huang M, Kusmartsev S et al (2004) Hyperactivation of STAT3 is involved in abnormal differentiation of dendritic cells in cancer. J Immunol 172:464–474
Soria G, Ben-Baruch A (2008) The inflammatory chemokines CCL2 and CCL5 in breast cancer. Cancer Lett 267:271–285
Yamashiro S, Takeya M, Nishi T et al (1994) Tumor-derived monocyte chemoattractant protein-1 induces intratumoral infiltration of monocyte-derived macrophage subpopulation in transplanted rat tumors. Am J Pathol 145:856–867
Rössner S, Voigtländer C, Wiethe C, Hänig J, Seifarth C, Lutz MB (2005) Myeloid dendritic cell precursors generated from bone marrow suppress T cell responses via cell contact and nitric oxide production in vitro. Eur J Immunol 35:3533–3544
Acknowledgments
This work was supported by NIH R01 CA104757 grant (M. H. Manjili) and Department of Defense Grant BC083048. We thank Julie Farnsworth for her expertise in cell sorting and immense dedication to furthering the research at our institution. Flow Cytometry supported in part by NIH Grant P30 CA16059. We gratefully acknowledge the support of VCU Massey Cancer Centre and the Commonwealth Foundation for Cancer Research.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Morales, J.K., Kmieciak, M., Knutson, K.L. et al. GM-CSF is one of the main breast tumor-derived soluble factors involved in the differentiation of CD11b-Gr1- bone marrow progenitor cells into myeloid-derived suppressor cells. Breast Cancer Res Treat 123, 39–49 (2010). https://doi.org/10.1007/s10549-009-0622-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-009-0622-8