Abstract
Estimating the natural history parameters of breast cancer not only elucidates the disease progression but also make contributions to assessing the impact of inter-screening interval, sensitivity, and attendance rate on reducing advanced breast cancer. We applied three-state and five-state Markov models to data on a two-yearly routine mammography screening in Finland between 1988 and 2000. The mean sojourn time (MST) was computed from estimated transition parameters. Computer simulation was implemented to examine the effect of inter-screening interval, sensitivity, and attendance rate on reducing advanced breast cancers. In three-state model, the MST was 2.02 years, and the sensitivity for detecting preclinical breast cancer was 84.83%. In five-state model, the MST was 2.21 years for localized tumor and 0.82 year for non-localized tumor. Annual, biennial, and triennial screening programs can reduce 53, 37, and 28% of advanced cancer. The effectiveness of intensive screening with poor attendance is the same as that of infrequent screening with high attendance rate. We demonstrated how to estimate the natural history parameters using a service screening program and applied these parameters to assess the impact of inter-screening interval, sensitivity, and attendance rate on reducing advanced cancer. The proposed method makes contribution to further cost-effectiveness analysis. However, these findings had better be validated by using a further long-term follow-up data.



Similar content being viewed by others
References
Tabar L, Fagerberg G, Chen HH, Duffy SW, Smart CR, Gad A, Smith RA (1995) Efficacy of breast cancer screening by age. Cancer 75:2507–2517
Chen HH, Duffy SW, Tabar L, Day NE (1997) Markov chain models for progression of breast cancer. Part I: tumour attributes and the preclinical screen-detectable phase. J Epidemiol Biostat 2:9–23
Chen HH, Duffy SW, Tabar L, Day NE (1997) Markov chain models for progression of breast cancer. Part II: prediction of outcomes for different screening regimes. J Epidemiol Biostat 2:25–35
Lai MS, Yen MF, Kuo HS, Koong SL, Chen THH, Duffy SW (1998) Efficacy of breast-cancer screening for female relatives of breast-cancer-index cases: Taiwan multicentre cancer screening (TAMCAS). Int J Cancer 78:21–26
Tabar L, Fagerberg G, Chen HH, Duffy SW, Smart CR, Gad A, Smith RA (1995) Efficacy of breast cancer screening by age. Cancer 1995:2507–2517
Weedon-Fekjær H, Vatten LJ, Aalen OO, Lindqvist B, Tretli S (2005) Estimating mean sojourn time and screening test sensitivity in breast cancer mammography screening: new results. J Med Screen 12:172–178
Sarkeala T, Anttila A, Forsman H, Luostarinen T, Saarenmaa I, Hakama M (2004) Process indicators from ten centres in the Finnish breast cancer screening programme from 1991 to 2000. Eur J Cancer 40:2116–2125. doi:10.1016/j.ejca.2004.06.017
Wu JC, Anttila A, Yen AM, Hakama M, Saarenmaa I, Sarkeala T, Malila N, Auvinen A, Chiu SY, Chen THH (2009) Evaluation of breast cancer service screening programme with a Bayesian approach: mortality analysis in a Finnish region. Breast Cancer Res Treat (in press). doi:10.1007/s10549-009-0604-x
Chen HH, Duffy SW, Tabar L (1996) A markov chain method to estimate the tumour progression rate from preclinical to clinical phase, sensitivity and positive predictive value for mammography in breast cancer screening. The Stat 45:307–317
Chen THH, Kuo HS, Yen MF, Lai MS, Tabar L, Duffy SW (2000) Estimation of sojourn time in chronic disease screening without data on interval cases. Biometrics 56:167–172
Sarkeala T, Hakama M, Saarenmaa I, Hakulinen T, Forsman H, Anttila A (2006) Episode sensitivity in association with process indicators in the Finnish breast cancer screening program. Int J Cancer 118:174–179. doi:10.1002/ijc.21310
Duffy SW, Day NE, Tabar L, Chen HH, Smith TC (1997) Markov models of breast tumor progression: some age-specific results. J Natl Cancer Inst Monogr 22:93–97
Anttila A, Koskela J, Hakama M (2002) Programme sensitivity and effectiveness of mammography service screening in Helsinki, Finland. J Med Screen 9:153–158
Duffy SW, Chen HH, Tabar L, Day NE (1995) Estimation of mean sojourn time in breast cancer screening using a Markov chain model of both entry to and exit from the preclinical detectable phase. Statist Med 14:1531–1543
Kay R (1986) A Markov model for analyzing cancer markers and disease states in survival studies. Biometrics 42:855–865
Acknowledgment
This research work was supported by the FiDiPro Research Project of Tampere School of Public Health Granted from Academy of Finland. It was also supported by the National Science Council of Taiwan (NSC 96-2628-B-002-096-Mr3).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendix
Appendix
Three-state Model
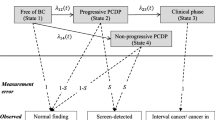
Let the stochastic process of disease natural history of breast cancer denoted by a random variable X(t), the outcome of which is defined by a state space W = {1,2,3}, where states 1, 2, and 3 stand for free of breast cancer, the PCDP, and clinical caner, respectively. In this three-state model, the transition probabilities from previous state (i) to current state (j) during time t can be represented by Table 6.
The application of this transition matrix to data on breast cancer screening has been described in full elsewhere [10]. The detailed likelihood functions for estimating the natural history parameters are decomposed by round of screens and detection modes.
Prevalent Screen
Suppose women invited to first screen (prevalent screen) at age m, the probabilities of having negative screening result (P s1_1) and positive screen-detected results (P s1_2) using transition probabilities and sensitivity (sen) and specificity (spe) are written as follows.
Those women who have negative screening results at first screen are composed of those who are actually disease free (true negative) or misclassified (false negative), whereas those women who have positive screening results at first screen consist of who are actually disease (true positive) or misclassified (false positive).
Incident Screen
Owing to false negative cases missed at prevalent screen, the underlying population for the second screen after the prevalent screen consist of disease free (true negative) and asymptomatic women missed at first screen with the respective proportions denoted by tn(1) (true negative at first screen) and fn(1) (false negative at first screen), respectively. The formulae of these two probabilities are expressed as follows.
Assume the false negative cases missed at first screen can be detected at the second screen, the probabilities of screen-negative women (P s2_1) and screen-detected cases (P s2_2) at the second screen and the probabilities of interval cancer (P s2_I) diagnosed before second screen and refuser cases (P s2_R) at second screen but diagnosed at time t after first screen are expressed as follows.
The first components of the Eqs. 5–7 delineate the progression of true negative subjects after prevalent screen and the second components give delineate the progression of false negative cases missed at prevalent screen. Note that, in the above formulae, t is the time interval between the prevalent and the second screen, inter-screening interval, or time after first screen for the refuser, and \( \Updelta t, \) say one month, which is an approximation to the instantaneous time (dt) used in the derivative of the probability density function. For example, the first component of the bracket on the right side of the Eq. 7, P 11(t − ∆t) × P 13(∆t) + P 12(t − ∆t) × P 23(∆t), which is called compound probability, is an approximation to dP 13(t)/dt. The merit of using compound probability has been described in Duffy et al. study [14] and Kay [15] as it can accommodate the rapid and slow progression of breast cancer.
Similar to the prevalent screen, the underlying population for the third screen after the second screen round is also composed of two categories, disease free and asymptomatic women missed at second screen, with the respective probabilities of tn(2) and fn(2) with formulae as follows.
The above formulae can be simplified as
The likelihood function for screen-detected cases, screen-negative women, and interval cancer at the third screen round can be derived in a similar manner by the incorporation of tn(2) and fn(2) into the likelihood function.
The Refuser Group
The probability of developing breast cancer for those who never come to screen \( \left( {P_{\text{NA}} } \right) \) is expressed as follows. In the following formula, m represents the age of the first invitation to the screening program, t 2 represents the time period from the first invitation to the year of the diagnosis of breast cancer.
The Eq. 13 is also applied to PSC (see Table 1).
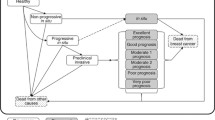
Five-state model
The likelihood functions using for the five-state model are developed in the same way as the three-state model. In the five-state model, the state space is changed as W = {1,2,3,4,5}, where states 1, 2, 3, 4, and 5 denote free of breast cancer, preclinical localized cancer, preclinical non-localized cancer, clinical localized cancer, and clinical non-localized cancer, respectively. The transition probabilities from state i to state j during time t can be represented by Table 7.
As in three-state model, the likelihood functions for the estimation of parameters are also decomposed by rounds of screen and detection modes. The details of likelihood functions are given with supplemental files and available online (http://homepage.ntu.edu.tw/~ntucbsc/tony_e.htm).
Rights and permissions
About this article
Cite this article
Wu, J.CY., Hakama, M., Anttila, A. et al. Estimation of natural history parameters of breast cancer based on non-randomized organized screening data: subsidiary analysis of effects of inter-screening interval, sensitivity, and attendance rate on reduction of advanced cancer. Breast Cancer Res Treat 122, 553–566 (2010). https://doi.org/10.1007/s10549-009-0701-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-009-0701-x