Abstract
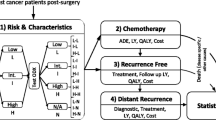
The 21-gene signature is validated as a good predictor of recurrence for lymph node-negative/positive, hormone receptor-positive, early-stage breast cancer in Japanese patient population. This study evaluates the cost-effectiveness of two scenarios designed to include the assay into Japan’s social health insurance benefit package: one for LN−, ER+, ESBC and another for LN−/+, ER+, ESBC. An economic decision tree and Markov model under Japan’s health system from the societal perspective is constructed with new evidence from the Japanese validation study. Incremental cost-effectiveness ratios are estimated as ¥384,828 (US$3,848) per QALY for the indication for LN− scenario and ¥568,533 (US$5,685) per QALY for the indication for LN−/+ scenario. Both are not more than the suggested social willingness-to-pay for one QALY gain from an innovative medical intervention in Japan, ¥5,000,000/QALY (US$50,000/QALY). Sensitivity analyses show that this result is plausibly robust, since ICERs do not exceed the threshold by various changes of assumptions made and values employed. In conclusion, the inclusion of the assay in Japan’s social health insurance benefit package for not only LN− diseases but also LN+ diseases is cost-effective. Such a decision can be justifiable as an efficient use of finite resources for health care.


Similar content being viewed by others
References
The Japanese Breast Cancer Society (2009) Zenkoku nyugan kanjya touroku chousa houkoku—zanteiban—dai 38 gou 2007 nenji shourei (National breast cancer registry report—provisional edition—No. 38 2007 cases). The Japanese Breast Cancer Society, Tokyo
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) (2005) Effects of chemotherapy and endocrine therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 365(9472):1687–1717
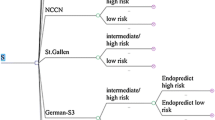
Goldhirsch A, Ingle JN, Gelber RD, Coates AS, Thürlimann B, Senn HJ, Panel members (2009) Thresholds for therapies: highlights of the St Gallen International Expert Consensus on the primary therapy of early breast cancer. Ann Oncol 20(8):1319–1329
Paik S, Shak S, Tang G, Kim C, Baker J, Cronin M, Baehner FL, Walker MG, Watson D, Park T, Hiller W, Fisher ER, Wickerham DL, Bryant J, Wolmark N (2004) A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med 351(27):2817–2826
Paik S, Tang G, Shak S, Kim C, Baker J, Kim W, Cronin M, Baehner FL, Watson D, Bryant J, Costantino JP, Geyer CE Jr, Wickerham DL, Wolmark N (2006) Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol 24(23):3726–3734
Habel LA, Shak S, Jacobs MK, Capra A, Alexander C, Pho M, Baker J, Walker M, Watson D, Hackett J, Blick NT, Greenberg D, Fehrenbacher L, Langholz B, Quesenberry CP (2006) A population-based study of tumor gene expression and risk of breast cancer death among lymph node-negative patients. Breast Cancer Res 8(3):R25
Harris L, Fritsche H, Mennel R, Norton L, Ravdin P, Taube S, Somerfield MR, Hayes DF, Bast RC Jr, American Society of Clinical Oncology (2007) American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol 25(33):5287–5312
National Comprehensive Cancer Network (2008) NCCN clinical practice guidelines in OncologyTM Breast Cancer V.2.2008
Albain KS, Barlow WE, Shak S, Hortobagyi GN, Livingston RB, Yeh IT, Ravdin P, Bugarini R, Baehner FL, Davidson NE, Sledge GW, Winer EP, Hudis C, Ingle JN, Perez EA, Pritchard KI, Shepherd L, Gralow JR, Yoshizawa C, Allred DC, Osborne CK, Hayes DF, Breast Cancer Intergroup of North America (2010) Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, estrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial. Lancet Oncol 11(1):55–65
Dowsett M, Cuzick J, Wale C, Forbes J, Mallon EA, Salter J, Quinn E, Dunbier A, Baum M, Buzdar A, Howell A, Bugarini R, Baehner FL, Shak S (2010) Prediction of risk of distant recurrence using the 21-gene recurrence score in node-negative and node-positive postmenopausal patients with breast cancer treated with anastrozole or tamoxifen: a TransATAC study. J Clin Oncol 28(11):1829–1834
Hornberger J, Cosler LE, Lyman GH (2005) Economic analysis of targeting chemotherapy using a 21-gene RT-PCR assay in lymph-node-negative, estrogen-receptor-positive, early-stage breast cancer. Am J Manag Care 11(5):313–324
Lyman GH, Cosler LE, Kuderer NM, Hornberger J (2007) Impact of a 21-gene RT-PCR assay on treatment decisions in early-stage breast cancer: an economic analysis based on prognostic and predictive validation studies. Cancer 109(6):1011–1018
Klang SH, Hammerman A, Liebermann N, Efrat N, Doberne J, Hornberger J (2010) Economic implications of 21-gene breast cancer risk assay from the perspective of an Israeli-managed health-care organization. Value Health 13(4):381–387
Tsoi DT, Inoue M, Kelly CM, Verma S, Pritchard KI (2010) Cost-effectiveness analysis of recurrence score-guided treatment using a 21-gene assay in early breast cancer. Oncologist 15(5):457–465
Kondo M, Hoshi SL, Ishiguro H, Yoshibayashi H, Toi M (2008) Economic evaluation of 21-gene reverse transcriptase-polymerase chain reaction assay in lymph-node-negative, estrogen-receptor-positive, early-stage breast cancer in Japan. Breast Cancer Res Treat 112(1):175–187
Toi M, Iwata H, Yamanaka T, Masuda N, Ohno S, Nakamura S, Nakayama T, Kashiwaba M, Kamigaki S, Kuroi K, the Japan Breast Cancer Research Group-Translational Research Group (2010) Clinical significance of the 21-gene signature (Oncotype DX) in hormone receptor-positive early stage primary breast cancer in the Japanese population. Cancer 116(13):3112–3118
Iwata H, Saeki T (2006) Current practices in breast cancer treatment in Japan—a questionnaire survey. Jpn J Breast Cancer 21(3):311–322
Garnock-Jones KP, Keating GM, Scott LJ (2010) Trastuzumab: a review of its use as adjuvant treatment in human epidermal growth factor receptor 2 (HER2)-positive early breast cancer. Drugs 70(2):215–239
Elkin EB, Weinstein MC, Winer EP, Kuntz KM, Schnitt SJ, Weeks JC (2004) HER-2 testing and trastuzumab therapy for metastatic breast cancer: a cost-effectiveness analysis. J Clin Oncol 22(5):854–863
Ministry of Health, Labour, Welfare (2007) The 20th life tables. Health and Welfare Statistics Association, Tokyo
Mouridsen H, Gershanovich M, Sun Y, Perez-Carrion R, Boni C, Monnier A, Apffelstaedt J, Smith R, Sleeboom HP, Jaenicke F, Pluzanska A, Dank M, Becquart D, Bapsy PP, Salminen E, Snyder R, Chaudri-Ross H, Lang R, Wyld P, Bhatnagar A (2003) Phase III study of letrozole versus tamoxifen as first-line therapy of advanced breast cancer in postmenopausal women: analysis of survival and update of efficacy from the International Letrozole Breast Cancer Group. J Clin Oncol 21(11):2101–2109
Hillner BE, Smith TJ (1991) Efficacy and cost effectiveness of adjuvant chemotherapy in women with node-negative breast cancer. A decision-analysis model. N Engl J Med 324(3):160–168
Smith I, Procter M, Gelber RD, Guillaume S, Feyereislova A, Dowsett M, Goldhirsch A, Untch M, Mariani G, Baselga J, Kaufmann M, Cameron D, Bell R, Bergh J, Coleman R, Wardley A, Harbeck N, Lopez RI, Mallmann P, Gelmon K, Wilcken N, Wist E, Sánchez Rovira P, Piccart-Gebhart MJ, HERA study team (2007) 2-Year follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer: a randomised controlled trial. Lancet 369(9555):29–36
Earle CC, Chapman RH, Baker CS, Bell CM, Stone PW, Sandberg EA, Neumann PJ (2000) Systematic overview of cost-utility assessments in oncology. J Clin Oncol 18(18):3302–3317
Cole BF, Gelber RD, Gelber S, Coates AS, Goldhirsch A (2001) Polychemotherapy for early breast cancer: an overview of the randomised clinical trials with quality-adjusted survival analysis. Lancet 358(9278):277–286
Gold MR, Siegel JE, Russell LB, Weinstein MC (eds) (1996) Cost-effectiveness in health and medicine. Oxford University Press, New York
Japan Society of Clinical Oncology (2005) Kouganzai tekiseishiyou no gaidorain: nyuugan (Guideline of appropriate use of anti cancer drugs: breast cancer). Int J Clin Oncol 10(Suppl.):15–55
Japanese Breast Cancer Society (2006) Kagakuteki konkyo ni motozuku nyuugan shinryo gaidorain: 1 yakubutsu ryouhou 2006 nenban (Evidence-based breast cancer care guideline: 1 drug treatments 2006 version). Kanehara Shuppan, Tokyo
Iwata H, Nakamura S, Toi M, Shin E, Masuda N, Ohno S, Takatsuka Y, Hisamatsu K, Yamazaki K, Kusama M, Kaise H, Sato Y, Kuroi K, Akiyama F, Tsuda H, Kurosumi M, Japan Breast Cancer Research Group (2005) Interim analysis of a phase II trial of cyclophosphamide, epirubicin and 5-fluorouracil (CEF) followed by docetaxel as preoperative chemotherapy for early stage breast carcinoma. Breast Cancer 12(2):99–103
Papaldo P, Ferretti G, Di Cosimo S, Giannarelli D, Marolla P, Lopez M, Cortesi E, Antimi M, Terzoli E, Carlini P, Vici P, Botti C, Di Lauro L, Naso G, Nisticò C, Mottolese M, Di Filippo F, Ruggeri EM, Ceribelli A, Cognetti F (2006) Does granulocyte colony-stimulating factor worsen anemia in early breast cancer patients treated with epirubicin and cyclophosphamide? J Clin Oncol 24(19):3048–3055
Kondo M, Hoshi SL, Toi M (2009) Economic evaluation of chemoprevention of breast cancer with tamoxifen and raloxifene among high-risk women in Japan. Br J Cancer 100(2):281–290
Shiroiwa T, Sung YK, Fukuda T, Lang HC, Bae SC, Tsutani K (2010) International survey on willingness-to-pay (WTP) for one additional QALY gained: what is the threshold of cost effectiveness? Health Econ 19(4):422–437
Paik S, Shak S, Tang G, Kim C, Baker J, Cronin M, Baehner FL, Walker MG, Watson D, Bryant J, Wolmark N (2004) Risk classification of breast cancer patients by the recurrence score assay: comparison to guidelines based on patient age, tumor size, and tumor grade. In: Abstracts of 27th annual San Antonio breast cancer symposium, Texas, 8–11, December 2004
Acknowledgments
The study was funded by Japan’s Ministry of Health, Labour and Welfare research grant, a study on the construction of algorithm of multimodality therapy with biomarkers for primary breast cancer by a formulation of decision making process, led by Masakazu Toi (H18-3JIGAN-IPPAN-007), and was also supported by Grant-in-Aid for Scientific Research (C) by Japan’s Ministry of Education, Culture, Sports, Science and Technology (No. 22590451).
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kondo, M., Hoshi, SL., Yamanaka, T. et al. Economic evaluation of the 21-gene signature (Oncotype DX®) in lymph node-negative/positive, hormone receptor-positive early-stage breast cancer based on Japanese validation study (JBCRG-TR03). Breast Cancer Res Treat 127, 739–749 (2011). https://doi.org/10.1007/s10549-010-1243-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-010-1243-y