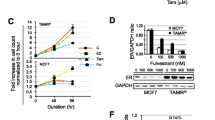
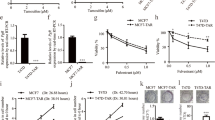
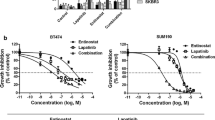
Abstract
Modulation of estrogen signaling is one of the most successful modalities for the treatment of estrogen receptor (ER)-positive breast cancer, yet de novo and acquired resistance are frequent. Recent data suggests that the induction of autophagy may play a considerable role in promoting tumor cell survival and resistance to anti-estrogen therapy. Hence, bypassing autophagy may offer a novel strategy to enhance the anti-tumor efficacy of anti-estrogens. Histone deacetylases (HDAC) are involved in the regulation of steroid hormone receptor mediated cell signaling and their inhibition potentiates the anti-tumor effects of anti-estrogens. However, the mechanism underlying this anti-tumor activity is poorly understood. In this report, we show that the addition of an HDAC inhibitor redirects the response of ER-positive breast cancer cells when treated with tamoxifen from growth arrest to apoptotic cell death. This redirection requires functional ER signaling and is mediated by a depletion of Bcl-2 and an induction of Bax and Bak, manifesting in cytochrome C release and PARP cleavage. With combined treatment, a subpopulation of cells is refractory to apoptosis and exhibit a strong induction of autophagy. Inhibition of autophagy in these cells, using siRNA directed against Beclin-1 or treatment with chloroquine, further promotes the induction of apoptosis. Thus, supporting prior reports that autophagy acts as a survival mechanism, our findings demonstrate that HDAC and autophagy inhibition directs autophagy-protected cells into apoptotic cell death, which may impair development of tamoxifen resistance.






Similar content being viewed by others
Abbreviations
- HDAC:
-
Histone deacetylase
- ER:
-
Estrogen receptor
- siRNA:
-
Small interfering RNA
- VPA:
-
Valproic acid
References
Thomas S, Munster PN (2009) Histone deacetylase inhibitor induced modulation of anti-estrogen therapy. Cancer Lett 280(2):184–191
Margueron R, Duong V, Castet A, Cavailles V (2004) Histone deacetylase inhibition and estrogen signalling in human breast cancer cells. Biochem Pharmacol 68(6):1239–1246
Huang BH, Laban M, Leung CH et al (2005) Inhibition of histone deacetylase 2 increases apoptosis and p21cip1/waf1 expression, independent of histone deacetylase 1. Cell Death Differ 12(4):395–404
Hrzenjak A, Moinfar F, Kremser ML et al (2006) Valproate inhibition of histone deacetylase 2 affects differentiation and decreases proliferation of endometrial stromal sarcoma cells. Mol Cancer Ther 5(9):2203–2210
Song J, Noh JH, Lee JH et al (2005) Increased expression of histone deacetylase 2 is found in human gastric cancer. Apmis 113(4):264–268
Zhu P, Martin E, Mengwasser J, Schlag P, Janssen KP, Gottlicher M (2004) Induction of hdac2 expression upon loss of apc in colorectal tumorigenesis. Cancer Cell 5(5):455–463
Halkidou K, Gaughan L, Cook S, Leung HY, Neal DE, Robson CN (2004) Upregulation and nuclear recruitment of hdac1 in hormone refractory prostate cancer. Prostate 59(2):177–189
Toh Y, Yamamoto M, Endo K et al (2003) Histone h4 acetylation and histone deacetylase 1 expression in esophageal squamous cell carcinoma. Oncol Rep 10(2):333–338
Osada H, Tatematsu Y, Saito H, Yatabe Y, Mitsudomi T, Takahashi T (2004) Reduced expression of class ii histone deacetylase genes is associated with poor prognosis in lung cancer patients. Int J Cancer 112(1):26–32
Zhang Z, Yamashita H, Toyama T et al (2005) Quantitation of hdac1 mrna expression in invasive carcinoma of the breast. Breast Cancer Res Treat 94(1):11–16
Krusche CA, Wulfing P, Kersting C et al (2005) Histone deacetylase-1 and -3 protein expression in human breast cancer: a tissue microarray analysis. Breast Cancer Res Treat 90(1):15–23
Duvic M, Talpur R, Ni X et al (2007) Phase ii trial of oral vorinostat (suberoylanilide hydroxamic acid, saha) for refractory cutaneous t-cell lymphoma (ctcl). Blood 109(1):31–39
Olsen EA, Kim YH, Kuzel TM et al (2007) Phase iib multicenter trial of vorinostat in patients with persistent, progressive, or treatment refractory cutaneous t-cell lymphoma. J Clin Oncol 25(21):3109–3115
Piekarz RL, Frye R, Turner M et al (2009) Phase ii multi-institutional trial of the histone deacetylase inhibitor romidepsin as monotherapy for patients with cutaneous t-cell lymphoma. J Clin Oncol 27(32):5410–5417
Sharma D, Blum J, Yang X, Beaulieu N, Macleod AR, Davidson NE (2005) Release of methyl cpg binding proteins and histone deacetylase 1 from the estrogen receptor alpha (er) promoter upon reactivation in er-negative human breast cancer cells. Mol Endocrinol 19(7):1740–1751
Yang X, Phillips DL, Ferguson AT, Nelson WG, Herman JG, Davidson NE (2001) Synergistic activation of functional estrogen receptor (er)-alpha by DNA methyltransferase and histone deacetylase inhibition in human er-alpha-negative breast cancer cells. Cancer Res 61(19):7025–7029
Fan J, Yin WJ, Lu JS et al (2008) Er alpha negative breast cancer cells restore response to endocrine therapy by combination treatment with both hdac inhibitor and dnmt inhibitor. J Cancer Res Clin Oncol 134(8):883–890
Bicaku E, Marchion DC, Schmitt M, Munster PN (2008) Selective inhibition of histone deacetylase 2 silences progesterone receptor mediated signaling. Cancer Res 68(5):1513–1519
Bursch W, Ellinger A, Kienzl H et al (1996) Active cell death induced by the anti-estrogens tamoxifen and ici 164 384 in human mammary carcinoma cells (mcf-7) in culture: The role of autophagy. Carcinogenesis 17(8):1595–1607
Bilir A, Altinoz MA, Erkan M, Ozmen V, Aydiner A (2001) Autophagy and nuclear changes in fm3a breast tumor cells after epirubicin, medroxyprogesterone and tamoxifen treatment in vitro. Pathobiology 69(3):120–126
Gozuacik D, Kimchi A (2004) Autophagy as a cell death and tumor suppressor mechanism. Oncogene 23(16):2891–2906
Qadir MA, Kwok B, Dragowska WH et al (2008) Macroautophagy inhibition sensitizes tamoxifen-resistant breast cancer cells and enhances mitochondrial depolarization. Breast Cancer Res Treat 112(3):389–403
Samaddar JS, Gaddy VT, Duplantier J et al (2008) A role for macroautophagy in protection against 4-hydroxytamoxifen-induced cell death and the development of antiestrogen resistance. Mol Cancer Ther 7(9):2977–2987
Gajdos C, Jordan VC (2002) Selective estrogen receptor modulators as a new therapeutic drug group: concept to reality in a decade. Clin Breast Cancer 2(4):272–281
Hodges-Gallagher L, Valentine CD, Bader SE, Kushner PJ (2006) Inhibition of histone deacetylase enhances the anti-proliferative action of antiestrogens on breast cancer cells and blocks tamoxifen-induced proliferation of uterine cells. Breast Cancer Res Treat 105(3):297–309
Scarlatti F, Bauvy C, Ventruti A et al (2004) Ceramide-mediated macroautophagy involves inhibition of protein kinase b and up-regulation of beclin 1. J Biol Chem 279(18):18384–18391
Levine B, Sinha S, Kroemer G (2008) Bcl-2 family members: dual regulators of apoptosis and autophagy. Autophagy 4(5):600–606
Galonek HL, Hardwick JM (2006) Upgrading the bcl-2 network. Nat Cell Biol 8(12):1317–1319
Kroemer G, Galluzzi L, Brenner C (2007) Mitochondrial membrane permeabilization in cell death. Physiol Rev 87(1):99–163
Shintani T, Klionsky DJ (2004) Autophagy in health and disease: a double-edged sword. Science 306(5698):990–995
Aita VM, Liang XH, Murty VV et al (1999) Cloning and genomic organization of beclin 1, a candidate tumor suppressor gene on chromosome 17q21. Genomics 59(1):59–65
Mathew R, Karantza-Wadsworth V, White E (2007) Role of autophagy in cancer. Nat Rev Cancer 7(12):961–967
Katayama M, Kawaguchi T, Berger MS, Pieper RO (2007) DNA damaging agent-induced autophagy produces a cytoprotective adenosine triphosphate surge in malignant glioma cells. Cell Death Differ 14(3):548–558
Amaravadi RK, Yu D, Lum JJ et al (2007) Autophagy inhibition enhances therapy-induced apoptosis in a myc-induced model of lymphoma. J Clin Invest 117(2):326–336
Abedin MJ, Wang D, Mcdonnell MA, Lehmann U, Kelekar A (2007) Autophagy delays apoptotic death in breast cancer cells following DNA damage. Cell Death Differ 14(3):500–510
Schoenlein PV, Periyasamy-Thandavan S, Samaddar JS, Jackson WH, Barrett JT (2009) Autophagy facilitates the progression of eralpha-positive breast cancer cells to antiestrogen resistance. Autophagy 5(3):400–403
Espina V, Mariani BD, Gallagher RI et al (2010) Malignant precursor cells pre-exist in human breast dcis and require autophagy for survival. PLoS One 5(4):e10240
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10549_2011_1364_MOESM1_ESM.tiff
Supplemental Figure 1. HDAC inhibitors potentiate the cytotoxicity of tamoxifen in T47D cells. T47D cells were treated with vehicle, C, 0.5 mM VPA, V, 10 μM 4OH-tamoxifen, T, or 0.5 mM VPA and 10 μM 4OH-tamoxifen, VT, and assayed for viability by dye exclusion assay (A) or apoptosis by scoring condensed and fragmented nuclei (B) at the indicated times. For viability and apoptosis, each condition was conducted in triplicate and presented as the average with error bars indicating the standard error of the mean. (TIFF 5680 kb)
Rights and permissions
About this article
Cite this article
Thomas, S., Thurn, K.T., Biçaku, E. et al. Addition of a histone deacetylase inhibitor redirects tamoxifen-treated breast cancer cells into apoptosis, which is opposed by the induction of autophagy. Breast Cancer Res Treat 130, 437–447 (2011). https://doi.org/10.1007/s10549-011-1364-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-011-1364-y