Abstract
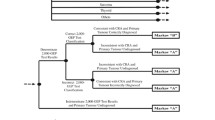
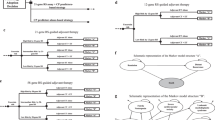
As gene expression profile (GEP) testing for breast cancer may provide additional prognostic information to guide the use of adjuvant chemotherapy, we examined the association between GEP testing and use of chemotherapy, serious chemotherapy-related adverse effects, and total charges during the 12 months following diagnosis. Medical record review was conducted for women age 30–64 years, with incident, non-metastatic, invasive breast cancer diagnosed 2006–2008 in a large, national health plan. Of 534 patients, 25.8% received GEP testing, 68.2% received chemotherapy, and 10.5% experienced a serious chemotherapy-related adverse effect. GEP testing was most commonly used in women at moderate clinical risk of recurrence (52.0 vs. 25.0% of low-risk women and 5.5% of high-risk). Controlling for the propensity to receive GEP testing, women who had GEP were less likely to receive chemotherapy (propensity adjusted odds ratio, 95% confidence interval 0.62, 0.39–0.99). Use of GEP was associated with more chemotherapy use among women at low risk based on clinical characteristics (OR = 42.19; CI 2.50–711.82), but less use among women with a high risk based on clinical characteristics (OR = 0.12; CI 0.03–0.47). Use of GEP was not associated with chemotherapy for the moderate risk group. There was no significant relationship between GEP use and either serious chemotherapy-associated adverse effects or total charges. While GEP testing was associated with an overall decrease in adjuvant chemotherapy, we did not find differences in serious chemotherapy-associated adverse events or charges during the 12 months following diagnosis.
Similar content being viewed by others
References
Jemal A, Siegel R, Xu J, Ward E (2010) Cancer statistics, 2010. CA Cancer J Clin 60(5):277–300
National Comprehensive Cancer Network (NCCN) Clinical practice guidelines in oncology: breast cancer v.1.20092009
Eifel P, Axelson JA, Costa J, Crowley J, Curran WJ Jr, Deshler A et al. (2001) National Institutes of Health Consensus Development Conference Statement: adjuvant therapy for breast cancer, November 1–3, 2000. J Natl Cancer Inst 93(13):979–989
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) (2005) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 365(9472):1687–1717
Warren JL, Yabroff KR, Meekins A, Topor M, Lamont EB, Brown ML (2008) Evaluation of trends in the cost of initial cancer treatment. J Natl Cancer Inst 100(12):888–897
Paik S, Shak S, Tang G, Kim C, Baker J, Cronin M et al. (2004) A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med 351(27):2817–2826
Goldstein LJ, Gray R, Badve S, Childs BH, Yoshizawa C, Rowley S et al. (2008) Prognostic utility of the 21-gene assay in hormone receptor-positive operable breast cancer compared with classical clinicopathologic features. J Clin Oncol 26(25):4063–4071
Albain K, Barlow W, Shak S, Hortobagyi G, Livingston R, Yeh I et al. (2007) Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal, node-positive, ER-positive breast cancer. San Antonio Breast Cancer Symposium, San Antonio, 2007
Paik S, Tang G, Shak S, Kim C, Baker J, Kim W et al. (2006) Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol 24(23):3726–3734
Sotiriou C, Pusztai L (2009) Gene-expression signatures in breast cancer. N Engl J Med 360(8):790–800
Genomic Health 2005 Annual Report. Available from http://files.shareholder.com/downloads/GHDX/1288212664x0x237978/DBCCB2E0-6584-496A-ACB1-D67DDA497401/2005AR.pdf. Accessed 11 June 2011
Breastcancer.org. OcotypeDX Test (2011) Available at http://www.breastcancer.org/symptoms/testing/types/oncotype_dx.jsp. Accessed 21 May 2011
Lyman GH, Cosler LE, Kuderer NM, Hornberger J (2007) Impact of a 21-gene RT-PCR assay on treatment decisions in early-stage breast cancer: an economic analysis based on prognostic and predictive validation studies. Cancer 109(6):1011–1018
Oestreicher N, Ramsey SD, Linden HM, McCune JS, van’t Veer LJ, Burke W et al. (2005) Gene expression profiling and breast cancer care: what are the potential benefits and policy implications? Genet Med 7(6):380–389
Hornberger J, Lyman GH, Chien R (2010) Economic implications of 21-gene recurrence score assay: US multicenter experience. J Clin Oncol 28(22):e382
Hornberger J, Cosler LE, Lyman GH (2005) Economic analysis of targeting chemotherapy using a 21-gene RT-PCR assay in lymph-node-negative, estrogen-receptor-positive, early-stage breast cancer. Am J Manag Care 11(5):313–324
Tsoi DT, Inoue M, Kelly CM, Verma S, Pritchard KI (2010) Cost-effectiveness analysis of recurrence score-guided treatment using a 21-gene assay in early breast cancer. Oncologist 15(5):457–465
Klang SH, Hammerman A, Liebermann N, Efrat N, Doberne J, Hornberger J (2010) Economic implications of 21-gene breast cancer risk assay from the perspective of an Israeli-managed health-care organization. Value Health 13(4):381–387
Gold JM, Najita JS, Lester S, Richardson AL, Morganstern DE, Chen WY et al. (2009) Personalizing treatment in early-stage breast cancer: the role of standard clinical factors and genomic information in adjuvant chemotherapy decision making. ASCO Annual Meeting, Orlando, 2009
Ademuyiwa FO, Miller A, O’Connor T, Edge SB, Thorat MA, Sledge GW et al (2011) The effects of oncotype DX recurrence scores on chemotherapy utilization in a multi-institutional breast cancer cohort. Breast Cancer Res Treat 126(3):797–802
Marchionni L, Wilson RF, Wolff AC, Marinopoulos S, Parmigiani G, Bass EB et al. (2008) Systematic review: gene expression profiling assays in early-stage breast cancer. Ann Intern Med 148(5):358–369
Webber EM, Lin JS, Whitlock EP (2010) Oncotype DX tumor gene expression profiling in stage II colon cancer. Application: prognostic, risk prediction. PLoS Curr 2:RRN1177
Hassett MJ, O’Malley AJ, Pakes JR, Newhouse JP, Earle CC (2006) Frequency and cost of chemotherapy-related serious adverse effects in a population sample of women with breast cancer. J Natl Cancer Inst 98(16):1108–1117
Liang SY, Phillips KA, Wang G, Keohane C, Armstrong J, Morris WM et al. (2011) Tradeoffs of using administrative claims and medical records to identify the use of personalized medicine for patients with breast cancer. Med Care 49(6):e1–e8
Albain KS, Barlow WE, Shak S, Hortobagyi GN, Livingston RB, Yeh IT et al. (2010) Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial. Lancet Oncol 11(1):55–65
Carlson B (2010) Payers try new approaches to manage molecular diagnostics. Biotechnol Healthcare 7(3):26–30
Deverka PA (2009) Pharmacogenomics, evidence, and the role of payers. Public Health Genomics 12(3):149–157
Klabunde CN, Potosky AL, Legler JM, Warren JL (2000) Development of a comorbidity index using physician claims data. J Clin Epidemiol 53(12):1258–1267
Adams JL, Mehrotra A, Thomas JW, McGlynn EA (2010) Physician cost profiling–reliability and risk of misclassification. N Engl J Med 362(11):1014–1021
Cepeda MS, Boston R, Farrar JT, Strom BL (2003) Comparison of logistic regression versus propensity score when the number of events is low and there are multiple confounders. Am J Epidemiol 158(3):280–287
Lo SS, Mumby PB, Norton J, Rychlik K, Smerage J, Kash J et al. (2010) Prospective multicenter study of the impact of the 21-gene recurrence score assay on medical oncologist and patient adjuvant breast cancer treatment selection. J Clin Oncol 28(10):1671–1676
Cardoso F, Van’t Veer L, Rutgers E, Loi S, Mook S, Piccart-Gebhart MJ (2008) Clinical application of the 70-gene profile: the MINDACT trial. J Clin Oncol 26(5):729–735
Stukel TA, Fisher ES, Wennberg DE, Alter DA, Gottlieb DJ, Vermeulen MJ (2007) Analysis of observational studies in the presence of treatment selection bias: effects of invasive cardiac management on AMI survival using propensity score and instrumental variable methods. JAMA 297(3):278–285
Kerlikowske K, Cook AJ, Buist DS, Cummings SR, Vachon C, Vacek P et al. (2010) Breast cancer risk by breast density, menopause, and postmenopausal hormone therapy use. J Clin Oncol 28(24):3830–3837
Acknowledgments
The study was supported by a grant from the National Cancer Institute (P01CA130818). Drs. Haas, Phillips, and Liang received funding from a research grant from the Aetna Foundation for earlier related research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Haas, J.S., Liang, SY., Hassett, M.J. et al. Gene expression profile testing for breast cancer and the use of chemotherapy, serious adverse effects, and costs of care. Breast Cancer Res Treat 130, 619–626 (2011). https://doi.org/10.1007/s10549-011-1628-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-011-1628-6