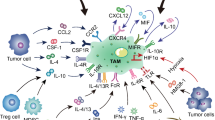
Abstract
Tumor-Associated Macrophages (TAM) represent the major inflammatory component of the stroma of many tumors, able to affect different aspects of the neoplastic tissue. Many observations indicate that TAM express several M2-associated protumoral functions, including promotion of angiogenesis, matrix remodelling and suppression of adaptive immunity. The protumoral role of TAM in cancer is further supported by clinical studies that found a correlation between the high macrophage content of tumors and poor patient prognosis and by evidence showing that long-term use of non-steroidal anti-inflammatory drugs reduces the risk of several cancers. Here, we discuss evidence supporting the view that TAM represent a unique and distinct M2-skewed myeloid population and a potential target of anti-cancer therapy.
Similar content being viewed by others
References
Balkwill, F., & Mantovani, A. (2001). Inflammation and cancer: Back to Virchow? Lancet, 357, 539–545.
Mantovani, A., Sozzani, S., Locati, M., Allavena, P., & Sica, A. (2002). Macrophage polarization: Tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends in Immunology, 23, 549–555.
Paik, S., Shak, S., Tang, G., Kim, C., Baker, J., Cronin, M., et al. (2004). A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. New England Journal of Medicine, 351, 2817–2826.
Koehne, C. H. (2004). Dubois RN: COX-2 inhibition and colorectal cancer. Seminars in Oncology, 31, 12–21.
Voronov, E., Shouval, D. S., Krelin, Y., Cagnano, E., Benharroch, D., Iwakura, Y., et al. (2003). IL-1 is required for tumor invasiveness and angiogenesis. Proceedings of the National Academy of Sciences of the United States of America, 100, 2645–2650.
Wyckoff, J., Wang, W., Lin, E. Y., Wang, Y., Pixley, F., Stanley, E. R., et al. (2004). A paracrine loop between tumor cells and macrophages is required for tumor cell migration in mammary tumors Cancer Research, 64, 7022–7029.
Pikarsky, E., Porat, R. M., Stein, I., Abramovitch, R., Amit, S., Kasem, S., et al. (2004). NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature, 431, 461–466.
Greten, F. R., Eckmann, L., Greten, T. F., Park, J. M., Li, Z. W., Egan, L. J., et al. (2004). IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell, 118, 285–296.
Coussens, L. M., & Werb, Z. (2002). Inflammation and cancer. Nature, 420, 860–867.
Mantovani, A., Sica, A., Sozzani, S., Allavena, P., Vecchi, A., & Locati, M. (2004). The chemokine system in diverse forms of macrophage activation and polarization. Trends in Immunology, 25, 677–686.
Sher, A., Pearce, E., & Kaye, P. (2003). Shaping the immune response to parasites: Role of dendritic cells. Current Opinion in Immunology, 15, 421–429.
Balkwill, F., Charles, K. A., & Mantovani, A. (2005). Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell, 7, 211–217.
Gordon, S. (2003). Alternative activation of macrophages. Natural Reviews. Immunology, 3, 23–35.
Mosser, D. M. (2003). The many faces of macrophage activation. Journal of Leukocyte Biology, 73, 209–212.
Goerdt, S., & Orfanos, C. E. (1999). Other functions, other genes: Alternative activation of antigen-presenting cells. Immunity, 10, 137–142.
Mantovani, A., Bottazzi, B., Colotta, F., Sozzani, S., & Ruco, L. (1992). The origin and function of tumor-associated macrophages. Immunology Today, 13, 265–270.
Hanahan, D., & Weinberg, R. A. (2000). The hallmarks of cancer. Cell, 100, 57–70.
Karin, M., Cao, Y., Greten, F. R., & Li, Z. W. (2002). NF-kappaB in cancer: From innocent bystander to major culprit. Nature Reviews. Cancer, 2, 301–310.
Mantovani, A. (2005). Cancer: Inflammation by remote control. Nature, 435, 752–753.
Luo, J. L., Maeda, S., Hsu, L. C., Yagita, H., & Karin, M. (2004). Inhibition of NF-kappaB in cancer cells converts inflammation-induced tumor growth mediated by TNFalpha to TRAIL-mediated tumor regression. Cancer Cell, 3, 297–305.
Andela, V. B., Schwarz, E. M., Puzas, J. E., O'Keefe, R. J., & Rosier, R. N. (2000). Tumor metastasis and the reciprocal regulation of prometastatic and antimetastatic factors by nuclear factor kappaB. Cancer Research, 60, 6557–6562.
Pahl, H. L. (1999). Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene, 18, 6853–6866.
Huang, S., Pettaway, C. A., Uehara, H., Bucana, C. D., & Fidler, I. J. (2001). Blockade of NF-kappaB activity in human prostate cancer cells is associated with suppression of angiogenesis, invasion, and metastasis. Oncogene, 20, 4188–4197.
Baldwin, A. S. Jr. (1996). The NF-kappa B and I kappa B proteins: New discoveries and insights. Annual Review of Immunology, 14, 649–683.
Ghosh, S., May, M. J., & Kopp, E. B. (1998). NF-kappa B and Rel proteins: Evolutionarily conserved mediators of immune responses. Annual Review of Immunology, 16, 225–260.
Baldwin, A. S. (2001). Control of oncogenesis and cancer therapy resistance by the transcription factor NF-kappaB. Journal of Clinical Investigation, 107, 241–246.
Wolf, J. S., Chen, Z., Dong, G., Sunwoo, J. B., Bancroft, C. C., Capo, D. E., et al. (2001). IL (interleukin)-1alpha promotes nuclear factor-kappaB and AP-1-induced IL-8 expression, cell survival, and proliferation in head and neck squamous cell carcinomas.Clinical Cancer Research, 7, 1812–1820.
Orlowski, R. Z., & Baldwin, A. S., Jr. (2002). NF-kappaB as a therapeutic target in cancer. Trends in Molecular Medicine, 8, 385–389.
Karin, M., Yamamoto, Y., & Wang, Q. M. (2004). The IKK NF-kappa B system: A treasure trove for drug development. Nat Rev Drug Discov, 3, 17–26.
Sica, A., Saccani, A., Bottazzi, B., Polentarutti, N., Vecchi, A., van Damme, J., et al. (2000). Autocrine production of IL-10 mediates defective IL-12 production and NF-kappa B activation in tumor-associated macrophages. Journal of Immunology, 164, 762–767.
Dinapoli, M. R., Calderon, C. L., & Lopez, D. M. (1996). The altered tumoricidal capacity of macrophages isolated from tumor-bearing mice is related to reduce expression of the inducible nitric oxide synthase gene. Journal of Experimental Medicine, 183, 1323–1329.
Ghosh, P., Komschlies, K. L., Cippitelli, M., Longo, D. L., Subleski, J., Ye, J., et al. (1995). Gradual loss of T-helper 1 populations in spleen of mice during progressive tumor growth. Journal of the National Cancer Institute, 87, 1478–1483.
Vaupel, P. (2004). The role of hypoxia-induced factors in tumor progression. Oncologist, 9, 10–17.
Semenza, G. L. (2003). Targeting HIF-1 for cancer therapy. Natural Reviews. Cancer, 3, 721–732.
Schlappack, O. K., Zimmermann, A., & Hill, R. P. (1991). Glucose starvation and acidosis: Effect on experimental metastatic potential, DNA content and MTX resistance of murine tumour cells. British Journal of Cancer, 64, 663–670.
Snyder, S. A., Lanzen, J. L., Braun, R. D., Rosner, G., Secomb, T. W., Biaglow, J., et al. (2001). Simultaneous administration of glucose and hyperoxic gas achieves greater improvement in tumor oxygenation than hyperoxic gas alone. International Journal of Radiation Oncology, Biology, Physics, 51, 494–506.
Walenta, S., Wetterling, M., Lehrke, M., Schwickert, G., Sundfor, K., Rofstad, E. K., et al. (2000). High lactate levels predict likelihood of metastases, tumor recurrence, and restricted patient survival in human cervical cancers. Cancer Research, 60, 916–921.
Sen, S., Zhou, H., Zhang, R. D., Yoon, D. S., Vakar-Lopez, F., Ito, S., et al. (2002). Amplification/overexpression of a mitotic kinase gene in human bladder cancer. Journal of the National Cancer Institute, 94, 1320–1329.
Koong, A. C., Denko, N. C., Hudson, K. M., Schindler, C., Swiersz, L., Koch, C., et al. (2000). Candidate genes for the hypoxic tumor phenotype. Cancer Research, 60, 883–887.
Czekay, R. P., Aertgeerts, K., Curriden, S. A., & Loskutoff, D. J. (2003). Plasminogen activator inhibitor-1 detaches cells from extracellular matrices by inactivating integrins. Journal of Cell Biology, 160, 781–791.
Talks, K. L., Turley, H., Gatter, K. C., Maxwell, P. H., Pugh, C. W., Ratcliffe, P. J., et al. (2000). The expression and distribution of the hypoxia-inducible factors HIF-1alpha and HIF-2alpha in normal human tissues, cancers, and tumor-associated macrophages. American Journal of Pathology, 157, 411–421.
Knowles, H., Leek, R., & Harris, A. L. (2004). Macrophage infiltration and angiogenesis in human malignancy. Novartis Foundation Symposium, 256, 189–200.
Cramer, T., Yamanishi, Y., Clausen, B. E., Forster, I., Pawlinski, R., Mackman, N., et al. (2003). HIF-1α is essential for myeloid cell-mediated inflammation. Cell, 112, 645–657.
Schioppa, T., Uranchimeg, B., Saccani, A., Biswas, S. K., Doni, A., Rapisarda, A., et al. (2003). Regulation of the chemokine receptor CXCR4 by hypoxia. Journal of Experimental Medicine, 198, 1391–1402.
Ceradini, D. J., Kulkarni, A. R., Callaghan, M. J., Tepper, O. M., Bastidas, N., Kleinman, M. E., et al. (2004). Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Natural Medicine, 10, 858–864.
Muller, A., Homey, B., Soto, H., Ge, N., Catron, D., Buchanan, M. E., et al. (2001). Involvement of chemokine receptors in breast cancer metastasis. Nature, 410, 50–56.
Pennacchietti, S., Michieli, P., Galluzzo, M., Mazzone, M., Giordano, S., & Comoglio, P. M. (2003). Hypoxia promotes invasive growth by transcriptional activation of the met protooncogene. Cancer Cell, 3, 347–361.
Giaccia, A., Siim, B. G., & Johnson, R. S. (2003). HIF-1 as a target for drug development. Nat Rev Drug Discov, 2, 803–811.
Folkman, J. (1995). Angiogenesis in cancer, vascular, rheumatoid and other disease. Natural Medicines, 1, 27–31.
Hanahan, D., & Folkman, J. (1996). Patterns of emerging mechanisms of the angiogenic switch during tumorigenesis. Cell, 86, 353–364.
Crowther, M., Brown, N. J., Bishop, E. T., & Lewis, C. E. (2001). Microenvironmental influence on macrophage regulation of angiogenesis in wounds and malignant tumors. Journal of Leukocyte Biology, 70, 478–490.
Salcedo, R., Wasserman, K., Young, H. A., Grimm, M. C., Howard, O. M., Anver, M. R., et al. (1999). Vascular endothelial growth factor and basic fibroblast growth factor induce expression of CXCR4 on human endothelial cells: In vivo neovascularization induced by stromal-derived factor-1alpha. American Journal of Pathology, 154, 1125–1135.
Cursiefen, C., Chen, L., Borges, L. P., Jackson, D., Cao, J., Radziejewski, C., et al. (2004). VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. Journal of Clinical Investigation, 113, 1040–1050.
Schoppmann, S. F., Birner, P., Stockl, J., Kalt, R., Ullrich, R., Caucig, C., et al. (2002). Tumor-associated macrophages express lymphatic endothelial growth factors and are related to peritumoral lymphangiogenesis. American Journal of Pathology, 161, 947–956.
Hotchkiss, K. A., Ashton, A. W., Klein, R. S., Lenzi, M. L., Zhu, G. H., & Schwartz, E. L. (2003). Mechanisms by which tumor cells and monocytes expressing the angiogenic factor thymidine phosphorylase mediate human endothelial cell migration. Cancer Research, 63, 527–533.
Azenshtein, E., Luboshits, G., Shina, S., Neumark, E., Shahbazian, D., Weil, M., et al. (2002). The CC chemokine RANTES in breast carcinoma progression: Regulation of expression and potential mechanisms of promalignant activity. Cancer Research, 62, 1093–1102.
Haghnegahdar, H., Du, J., Wang, D., Strieter, R. M., Burdick, M. D., Nanney, L. B., et al. (2000). The tumorigenic and angiogenic effects of MGSA/GRO proteins in melanoma. Journal of Leukocyte Biology, 67, 53–62.
Ueno, T., Toi, M., Saji, H., Muta, M., Bando, H., Kuroi, K., et al. (2000). Significance of macrophage chemoattractant protein-1 in macrophage recruitment, angiogenesis, and survival in human breast cancer. Clinical Cancer Research, 6, 3282–3289.
Bonizzi, G., & Karin, M. (2004). The two NF-kappaB activation pathways and their role in innate and adaptive immunity. Trends in Immunology, 25, 280–288.
Li, Q., & Verma, I. M. (2002). NF-kappaB regulation in the immune system. Nature reviews. Immunology, 2, 725–734.
Moore, R. J., Owens, D. M., Stamp, G., Arnott, C., Burke, F., East, N., et al. (1999). Tumour necrosis factor-a deficient mice are resistant to skin carcinogenesis. Natural Medicines, 5, 828–831.
Hagemann, T., Robinson, S. C., Schulz, M., Trumper, L., Balkwill, F. R., & Binder, C. (2004). Enhanced invasiveness of breast cancer cell lines upon co-cultivation with macrophages is due to TNF-α dependent up-regulation of matrix metalloproteases. Carcinogenesis, 25, 1543–1549.
Pollard, Jeffrey W. (2004). Tumour-educated macrophages promote tumour progression and metastasis. Nature Reviews Cancer, 4, 71–78.
Lin, E. Y., Nguyen, A. V., Russell, R. G., & Pollard, J. W. (2001). Colony-stimulating factor 1 promotes progression of mammary tumors to malignancy. Journal of Experimental Medicine, 193, 727–740.
Hiratsuka, S., Nakamura, K., Iwai, S., Murakami, M., Itoh, T., Kijima, H., et al. (2002). MMP9 induction by vascular endothelial growth factor receptor-1 is involved in lung-specific metastasis. Cancer Cell, 2, 289–300.
Grimshaw, M. J., Wilson, J. L., & Balkwill, F. R. (2002). Endothelin-2 is a macrophage chemoattractant: Implications for macrophage distribution in tumors. Europrean Journal of Immunology, 32, 2393–2400.
Grimshaw, M. J. (2005). Endothelins in breast tumour cell invasion. Cancer Letter, 222, 129–138.
Browatzki, M., Pfeiffer, C. A., Schmidt, J., & Kranzhofer, R. (2005). Endothelin-1 induces CD40 but not IL-6 in human monocytes via the proinflammatory transcription factor NF-kappaB. European Journal of Medical Research, 10, 197–201.
Spinella, F., Rosano, L., Di Castro, V., Natali, P. G., & Bagnato, A. (2002). Endothelin-1 induces vascular endothelial growth factor by increasing hypoxia-inducible factor-1alpha in ovarian carcinoma cells. Journal of Biological Chemistry, 277, 27850–27855.
Leek, R. D., & Harris, A. L. (2002). Tumor-associated macrophages in breast cancer. Journal of Mammary Gland Biology and Neoplasia, 7, 177–189.
O'Sullivan, C., & Lewis, C. E. (1994). Tumour-associated leucocytes: Friends or foes in breast carcinoma. Journal of Pathology, 172, 229–235.
Lin, E. Y., Gouon-Evans, V., Nguyen, A. V., & Pollard, J. W. (2002). The macrophage growth factor CSF-1 in mammary gland development and tumor progression. Journal of Mammary Gland Biology and Neoplasia, 7, 147–162.
Ishikawa, S., Egami, H., Kurizaki, T., Akagi, J., Tamori, Y., Yoshida, N., et al. (2003). Identification of genes related to invasion and metastasis in pancreatic cancer by cDNA representational difference analysis. Journal of Experimental and Clinical Cancer Research, 22, 299–306.
Eubank, T. D., Galloway, M., Montague, C. M., Waldman, W. J., Marsh, C. B. (2003). M-CSF induces vascular endothelial growth factor production and angiogenic activity from human monocytes. Journal of Immunology, 171, 2637–2643.
Hildenbrand, R., Dilger, I., Horlin, A., & Stutte, H. J. (1995). Urokinase and macrophages in tumour angiogenesis. British Journal of Cancer, 72, 818–823.
Klimetzek, V., & Sorg, C. (1977). Lymphokine-induced secretion of plasminogen activator by murine macrophages. European Journal of Immunology, 7, 185–187.
Ahmed, F., Wyckoff, J., Lin, E. Y., Wang, W., Wang, Y., Hennighausen L., et al. (2002). GFP expression in the mammary gland for imaging of mammary tumor cells in transgenic mice. Cancer Research, 62, 7166–7169.
Goswami, S., Sahai, E., Wyckoff, J. B., Cammer, M., Cox, D., Pixley, F. J., et al. (2005). Macrophages promote the invasion of breast carcinoma cells via a colony-stimulating factor-1/epidermal growth factor paracrine loop. Cancer Research, 65, 5278–5283.
Coussens, L. M., Tinkle, C. L., Hanahan, D., & Werb, Z. (2000). MMP-9 supplied by bone marrow-derived cells contributes to skin carcinogenesis. Cell, 103, 481–490.
Locati, M., Deuschle, U., Massardi, M. L., Martinez, F. O., Sironi, M., Sozzani, S., et al. (2002). Analysis of the gene expression profile activated by the CC chemokine ligand 5/RANTES and by lipopolysaccharide in human monocytes. Journal of Immunology, 168, 3557–3562.
Bronte, V., & Zanovello, P. (2005). Regulation of immune responses by l-arginine metabolism. Nature Reviews. Immunology, 5, 641–654.
de Visser, K. E., Korets, L. V., & Coussens, L. M. (2005). De novo carcinogenesis promoted by chronic inflammation is B lymphocyte dependent. Cancer Cell, 7, 411–423.
Grohmann, U., Fallarino, F., & Puccetti, P. (2003). Tolerance, DCs and tryptophan: Much ado about IDO. Trends in Immunology, 24, 242–248.
Muller, A. J., DuHadaway, J. B., Donover, P. S., Sutanto-Ward, E., & Prendergast, G. C. (2005). Inhibition of indoleamine 2,3-dioxygenase, an immunoregulatory target of the cancer suppression gene Bin1, potentiates cancer chemotherapy. Natural Medicines, 11, 312–319.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mantovani, A., Schioppa, T., Porta, C. et al. Role of tumor-associated macrophages in tumor progression and invasion. Cancer Metastasis Rev 25, 315–322 (2006). https://doi.org/10.1007/s10555-006-9001-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10555-006-9001-7