Abstract
Introduction
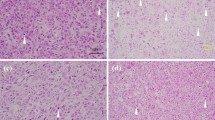
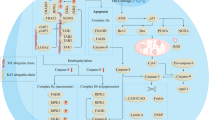
Radiotherapy, like most anticancer treatments, achieves its therapeutic effect by inducing different types of cell death in tumors.
Cell death markers and imaging modalities
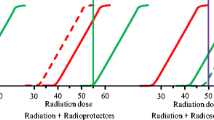
To evaluate treatment efficacy a variety of routine anatomical imaging modalities is used. However, changes in tumor physiology, metabolism and proliferation often precede these volumetric changes. Therefore, reliable biomarkers and imaging modalities that could assess treatment response more rapidly or even predict tumor responsiveness to treatment in an early phase would be very useful to identify responders and/or avoid ineffective, toxic therapies. A better understanding of cell death mechanisms following irradiation is essential for the development of such tools.
Cell death and available assays
In this review the most prominent types of radiation-induced cell death are discussed. In addition, the currently available assays to detect apoptosis, necrosis, mitotic catastrophe, autophagy and senescence in vitro and, if applicable, in vivo, are presented.





Similar content being viewed by others
References
Hanahan, D., & Weinberg, R. A. (2000). The hallmarks of cancer. Cell, 100, 57–70.
Kroemer, G., El-Deiry, W. S., Golstein, P., Peter, M. E., Vaux, D., Vandenabeele, P., et al. (2005). Classification of cell death: Recommendations of the Nomenclature Committee on Cell Death. Cell Death and Differentiation, 12, 1463–1467.
Hengartner, M. O. (2000). The biochemistry of apoptosis. Nature, 407, 770–776.
Wang, X. (2001). The expanding role of mitochondria in apoptosis. Genes & Development, 15, 2922–2233.
Puthalakath, H., & Strasser, A. (2002). Keeping killers on a tight leash: Transcriptional and post-translational control of the pro-apoptotic activity of BH3-only proteins. Cell Death and Differentiation, 9, 505–512.
Zong, W. X., Lindsten, T., Ross, A. J., MacGregor, G. R., & Thompson, C. B. (2001). BH3-only proteins that bind pro-survival Bcl-2 family members fail to induce apoptosis in the absence of Bax and Bak. Genes & Development, 15, 1481–1486.
Wei, M. C., Lindsten, T., Mootha, V. K., Weiler, S., Gross, A., Ashiya, M., et al. (2000). tBID, a membrane-targeted death ligand, oligomerizes BAK to release cytochrome c. Genes & Development, 14, 2060–2071.
Desagher, S., Osen-Sand, A., Nichols, A., Eskes, R., Montessuit, S., Lauper, S., et al. (1999). Bid-induced conformational change of Bax is responsible for mitochondrial cytochrome c release during apoptosis. Journal of Cell Biology, 144, 891–901.
Antonsson, B., Montessuit, S., Sanchez, B., & Martinou, J. C. (2001). Bax is present as a high molecular weight oligomer/complex in the mitochondrial membrane of apoptotic cells. Journal of Biological Chemistry, 276, 11615–11623.
Cheng, E. H., Wei, M. C., Weiler, S., Flavell, R. A., Mak, T. W., Lindsten, T., et al. (2001). BCL-2, BCL-X(L) sequester BH3 domain-only molecules preventing BAX- and BAK-mediated mitochondrial apoptosis. Molecular Cell, 8, 705–711.
Letai, A., Bassik, M. C., Walensky, L. D., Sorcinelli, M. D., Weiler, S., & Korsmeyer, S. J. (2002). Distinct BH3 domains either sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeutics. Cancer Cell, 2, 183–192.
Datta, R., Kojima, H., Banach, D., Bump, N. J., Talanian, R. V., Alnemri, E. S., et al. (1997). Activation of a CrmA-insensitive, p35-sensitive pathway in ionizing radiation-induced apoptosis. Journal of Biological Chemistry, 272, 1965–1969.
Kataoka, T., Schröter, M., Hahne, M., Schneider, P., Irmler, M., Thome, M., et al. (1998). FLIP prevents apoptosis induced by death receptors but not by perforin/granzyme B, chemotherapeutic drugs, and gamma irradiation. Journal of Immunology, 161, 3936–3942.
Tepper, A. D., de Vries, E., van Blitterswijk, W. J., & Borst, J. (1999). Ordering of ceramide formation, caspase activation, and mitochondrial changes during CD95- and DNA damage-induced apoptosis. Journal of Clinical Investigation, 103, 971–978.
Sentman, C. L., Shutter, C. L., Hockenberry, D., Kanagaa, O., & Korsmeyer, S. J. (1991). Bcl-2 inhibits multiple forms of apoptosis but not negative selection in thymocytes. Cell, 67, 879–888.
Strasser, A., Harris, A. W., Jacks, T., & Cory, S. (1994). DNA damage can induce apoptosis in proliferating lymphoid cells via p53-independent mechanisms inhibitable by Bcl-2. Cell, 79, 189–192.
Miyashita, T., Kralewski, S., Krajewska, M., Wang, H. G., Lin, H. K., Liebermann, D. A., et al. (1994). Tumor suppressor p53 is a regulator of bcl-2 and bax gene expression in vitro and in vivo. Oncogene, 9, 1799–1805.
Miyashita, T., & Reed, J. C. (1995). Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell, 80, 293–299.
Fei, P., Bernhard, E. J., & El-Deiry, W. S. (2002). Tissue-specific induction of p53 targets in vivo. Cancer Research, 62, 7316–7327.
Mihara, M., Erster, S., Zaika, A., Petrenko, O., Chittenden, T., Pancoska, P., et al. (2003). p53 has a direct apoptogenic role at the mitochondria. Molecular Cell, 11, 577–590.
Friesen, C., Herr, I., Krammer, P. H., & Debatin, K. M. (1996). Involvement of the CD95 (APO-1/Fas) receptor/ligand system in drug-induced apoptosis in leukemia cells. Nature Medicine, 2, 574–577.
Müller, M., Strand, S., Hug, H., Heinemann, E. M., Walczak, H., Hofmann, W. J., et al. (1997). Drug-induced apoptosis in hepatoma cells is mediated by the CD95 (APO-1/Fas) receptor/ligand system and involves activation of wild-type p53. Journal of Clinical Investigation, 99, 403–413.
Belka, C., Schmid, B., Marini, P., Durand, E., Rudner, J., Faltin, H., et al. (2001). Sensitization of resistant lymphoma cells to irradiation-induced apoptosis by the death ligand TRAIL. Oncogene, 20, 2190–2196.
Zong, W. X., Ditsworth, D., Bauer, D. E., Wang, Z. Q., & Thompson, C. B. (2004). Alkylating DNA damage stimulates a regulated form of necrotic cell death. Genes & Development, 18, 1272–1282.
Sun, X., Li, Y., Li, W., Zhang, B., Wang, A. J., Sun, J., et al. (2006). Selective induction of necrotic cell death in cancer cells by beta-lapachone through activation of DNA damage response pathway. Cell Cycle, 5, 2029–2035.
Takai, H., Tominaga, K., Motoyama, N., Minamishima, Y. A., Nagahama, H., Tsukiyama, T., et al. (2000). Aberrant cell cycle checkpoint function and early embryonic death in Chk1(−/−) mice. Genes & Development, 14, 1439–1447.
Ianzini, F., & Mackey, M. A. (1998). Delayed DNA damage associated with mitotic catastrophe following X-irradiation of HeLa S3 cells. Mutagenesis, 13, 337–344.
Blank, M., Lerenthal, Y., Mittelman, L., & Shiloh, Y. (2006). Condensin I recruitment and uneven chromatin condensation precede mitotic cell death in response to DNA damage. Journal of Cell Biology, 74, 195–206.
Kastan, M. B., Onyekwere, O., Sidransky, D., Vogelstein, B., & Craig, R. (1991). Participation of p53 protein in the cellular response to DNA damage. Cancer Research, 51, 6304–6311.
Wang, C. W., & Klionsky, D. J. (2003). The molecular mechanism of autophagy. Molecular Medicine, 9, 65–76.
Paglin, S., Hollister, T., Delohery, T., Hackett, N., McMahill, M., Sphicas, E., et al. (2001). A novel response of cancer cells to radiation involves autophagy and formation of acidic vesicles. Cancer Research, 61, 439–444.
Paglin, S., & Yahalom, J. (2006). Pathways that regulate autophagy and their role in mediating tumor response to treatment. Autophagy, 2, 291–293.
Levine, B., & Klionsky, D. J. (2004). Development by self-digestion: Molecular mechanisms and biological functions of autophagy. Developmental Cell, 6, 463–747.
Degenhardt, K., Mathew, R., Beaudoin, B., Bray, K., Anderson, D., Chen, G., et al. (2006). Autophagy promotes tumor cell survival and restricts necrosis, inflammation, and tumorigenesis. Cancer Cell, 10, 51–64.
Kelekar, A. (2005). Autophagy. Annals of the New York Academy of Sciences, 1066, 259–271.
Campisi, J. (2000). Cancer, aging and cellular senescence. In Vivo, 14, 183–188.
Roninson, I. B. (2003). Tumor cell senescence in cancer treatment. Cancer Research, 63, 2705–2715.
Verheij, M., van Blitterswijk, W. J., & Bartelink, H. (1998). Radiation-induced apoptosis: The ceramide-SAPK signaling pathway and clinical aspects. Acta Oncológica, 37, 575–581.
Chapman, J. D., & Anderson, P. R. (1999). Predicting and overcoming the radioresistance of individual tumors. International Journal of Radiation Oncology, Biology, Physics, 44, 477–479.
Ong, F., Moonen, L. M. F., Gallee, M. P. W., ten Bosch, C., Zerp, S. F., Hart, A. A. M., et al. (2001). Prognostic factors in transitional cell cancer of the bladder: An emerging role for Bcl-2 and p53. Radiotherapy and Oncology, 61, 169–175.
Gerke, V., & Moss, S. E. (2002). Annexins: From structure to function. Physiological Reviews, 82, 331–371.
Andree, H. A., Stuart, M. C., Hermens, W. T., Reutelingsperger, C. P., Hemker, H. C., Frederik, P. M., et al. (1992). Clustering of lipid-bound annexin V may explain its anticoagulant effect. Journal of Biological Chemistry, 267, 17907–17912.
Ahn, N. G., Teller, D. C., Bienkowski, M. J., McMullen, B. A., Lipkin, E. W., & de Haen, C. (1988). Sedimentation equilibrium analysis of five lipocortin-related phospholipase A2 inhibitors from human placenta. Evidence against a mechanistically relevant association between enzyme and inhibitor. Journal of Biological Chemistry, 263, 18657–18663.
van Heerde, W. L., de Groot, P. G., & Reutelingsperger, C. P. (1995). The complexity of the phospholipid binding protein Annexin V. Thrombosis and Haemostasis, 73, 172–179.
Sun, J., Bird, P., & Salem, H. H. (1993). Interaction of annexin V and platelets: Effects on platelet function and protein S binding. Thrombosis Research, 69, 289–296.
Sugimura, M., Donato, R., Kakkar, V. V., & Scully, M. F. (1994). Annexin V as a probe of the contribution of anionic phospholipids to the procoagulant activity of tumour cell surfaces. Blood Coagulation & Fibrinolysis, 5, 365–373.
Blankenberg, F. G., Katsikis, P. D., Tait, J. F., Davis, R. E., Naumovski, L., Ohtsuki, K., et al. (1999). Imaging of apoptosis (programmed cell death) with 99mTc annexin V. Journal of Nuclear Medicine, 40, 184–191.
Hofstra, L., Liem, I. H., Dumont, E. A., Boersma, H. H., van Heerde, W. L., Doevendans, P. A., et al. (2000). Visualisation of cell death in vivo in patients with acute myocardial infarction. Lancet, 356, 209–212.
Blankenberg, F. G., Robbins, R. C., Stoot, J. H., Vriens, P. W., Berry, G. J., Tait, J. F., et al. (2000). Radionuclide imaging of acute lung transplant rejection with annexin V. Chest, 117, 834–840.
Lorberboym, M., Blankenberg, F. G., Sadeh, M., & Lampl, Y. (2006). In vivo imaging of apoptosis in patients with acute stroke: Correlation with blood–brain barrier permeability. Brain Research, 1103, 13–19.
Belhocine, T., Steinmetz, N., Hustinx, R., Bartsch, P., Jerusalem, G., Seidel, L., et al. (2002). Increased uptake of the apoptosis-imaging agent (99m)Tc recombinant human Annexin V in human tumors after one course of chemotherapy as a predictor of tumor response and patient prognosis. Clinical Cancer Research, 8, 2766–2774.
Haas, R., de Jong, D., Valdés Olmos, R. A., Zerp, S. F., van den Heuvel, I., Bartelink, H., et al. (2004). In vivo imaging of radiation-induced apoptosis by 99mTc-annexin-V scintigraphy in follicular lymphoma patients. International Journal of Radiation Oncology, Biology, Physics, 59, 782–787.
Dubray, B., Breton, C., Delic, J., Klijanienko, J., Maciorowski, Z., Vielh, P., et al. (1997). In vitro radiation-induced apoptosis and tumour response to radiotherapy: A prospective study in patients with non-Hodgkin lymphomas treated by low-dose irradiation. International Journal of Radiation Biology, 72, 759–760.
Verheij, M., & Bartelink, H. (2000). Radiation-induced apoptosis. Cell and Tissue Research, 301, 133–142.
Kartachova, M., Haas, R. L. M., Valdés Olmos, R. A., Hoebers, F. J. P., van Zandwijk, N., & Verheij, M. (2004). In vivo imaging of apoptosis by 99m-annexin V scintigraphy: Visual analysis in relation to treatment response. Radiotherapy and Oncology, 72, 333–339.
Kartachova, M., van Zandwijk, N., Burgers, S., van Tinteren, H., Verheij, M., & Valdes Olmos, R. A. (2007). Prognostic significance of 99mTc Hynic-rh-Annexin V scintigraphy during platinum-based chemotherapy in advanced lung cancer. Journal of Clinical Oncology, 25, 2534–2539.
Kartachova, M., Verheij, M., van Eck, B., Hoefnagel, K., & Valdés Olmos, R. (2005). Methodological aspects and applications of in vivo imaging of apoptosis in oncology: An illustrative review. Current Medical Imaging Review, 1, 221–228.
Kartachova, M., Valdes Olmos, R. A., Haas, R. L. M., Hoebers, F. J. P., van den Brekel, M. W., van Zandwijk, N., et al. (2006). Mapping of treatment-induced apoptosis in normal structures: 99mTc hynic-rh-annexin V SPECT and CT image fusion. European Journal of Nuclear Medicine and Molecular Imaging, 33, 893–899.
Faust, A., Wagner, S., Law, M. P., Hermann, S., Schnockel, U., Keul, P., et al. (2007). The nonpeptidyl caspase binding radioligand (S)-1-(4-(2-[18F]fluoroethoxy)-benzyl)-5-[1-(2-methoxymethylpyrrolidinyl)sulfonyl]isatin [18F]CbR) as potential positron emission tomography-compatible apoptosis imaging agent. Quarterly Journal of Nuclear Medicine and Molecular Imaging, 51, 67–73.
Del Vecchio, S., Zannetti, A., Aloj, L., Caraco, C., Ciarmiello, A., & Salvatore, M. (2003). Inhibition of early 99mTc-MIBI uptake by Bcl-2 anti-apoptotic protein overexpression in untreated breast carcinoma. European Journal of Nuclear Medicine and Molecular Imaging, 30, 879–887.
Borst, G. R., Belderbos, J. S., Boellaard, R., Comans, E. F., De Jaeger, K., Lammertsma, A. A., et al. (2005). Standardised FDG uptake: A prognostic factor for inoperable non-small cell lung cancer. European Journal of Cancer, 41, 1533–1541.
Martinet, W., De Meyer, G. R., Andries, L., Herman, A. G., & Kockx, M. M. (2006). Detection of autophagy in tissue by standard immunohistochemistry: Possibilities and limitations. Autophagy, 2, 55–57.
Itahana, K., Campisi, J., & Dimri, G. P. (2007). Methods to detect biomarkers of cellular senescence: The senescence-associated beta-galactosidase assay. Methods in Molecular Biology, 371, 21–31.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Verheij, M. Clinical biomarkers and imaging for radiotherapy-induced cell death. Cancer Metastasis Rev 27, 471–480 (2008). https://doi.org/10.1007/s10555-008-9131-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10555-008-9131-1