Abstract
Background
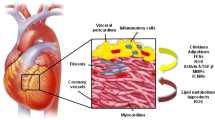
Epicardial adipose tissue (Epi) is a fat depot which is closely apposed on the myocardium. Several lines of evidence suggest that it is not only a lipid-storing depot, but also an active endocrine organ which secrets inflammatory cytokines and chemolines as suggested for other types of visceral fat.
Objectives and methods
We selected guinea pigs which have been shown to expand Epi with age, to investigate the expansion and properties of Epi and its impact on cardiac structure and function in detail.
Results
The amount of epicardial fat increases rapidly with age and accumulates at the aortic arch. It extends over the anterior surface of both ventricles and along the anterior and posterior branches of the coronary arteries. It also expands within the epicardium. The pattern of cytokines released by Epi is altered with age showing an up- and down-regulation of a variety of the 120 cytokines analyzed. Most prominently changed are IGFBP-4 and TIMP-2, whereas the release of adiponectin is not modified by age.
Conclusions
The amount of Epi is closely correlated to the amount of other types of visceral fat, to insulin resistance and other features of the metabolic syndrome, but also to cardiac hypertrophy and cardiac dysfunction. The data provide evidence that the guinea pig heart is a suitable model to analyze the interactions between Epi, heart vessels and muscle tissue. It allows identifying the influence of nutritional and metabolic alterations on the complexity of the network of locally released mediators for heart structure and function. A deeper understanding of this animal model may be helpful to analyze the interactions between Epi and the myocardium in humans—where the availability of tissue and the possibilities to modify nutritional and metabolic influences on heart are restricted—and the impact of Epi on cardiovascular risk.






Similar content being viewed by others
References
Rabkin SW. Epicardial fat: properties, function and relationship to obesity. Obes Rev. 2007;8:253–61.
Sacks HS, Fain JN. Human epicardial adipose tissue: a review. Am Heart J. 2007;153:907–17.
Wellen KE, Hotamisligil GS. Obesity-induced inflammatory changes in adipose tissue. J Clin Invest. 2003;112:1785–8.
Xu H, Barnes GT, Yang Q, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–30.
Willis RA. The anatomic examination of the body of Thomas Par. The works of William Harvey. London: Sydenham Society; 1847. p. 587–92.
Bedford E. The story of fatty heart. A disease of Victorian times. Brit Heart J. 1972;34:23–8.
Iacobellis G, Corradi D, Sharma AM. Epicardial adipose tissue: anatomic, biomolecular and clinical relationships with the heart. Nat Clin Pract Cardiovasc Med. 2005;2:536–43.
Iacobellis G, Ribaudo MC, Assael F, et al. Echocardiographic epicardial adipose tissue is related to anthropometric and clinical parameters of metabolic syndrome: a new indicator of cardiovascular risk. J Clin Endocrinol Metab. 2003;88:5163–8.
Iacobellis G, Assael F, Ribaudo MC, et al. Epicardial fat from echocardiography: a new method for visceral adipose tissue prediction. Obes Res. 2003;11:304–10.
Iacobellis G. Imaging of visceral adipose tissue: an emerging diagnostic tool and therapeutic target. Curr Drug Targets Cardiovasc Haematol Disord. 2005;5:345–53.
Iacobellis G, Leonetti F. Epicardial adipose tissue and insulin resistance in obese subjects. J Clin Endocrinol Metab. 2005;90:6300–2.
Iacobellis G, Ribaudo MC, Zappaterreno A, et al. Relation between epicardial adipose tissue and left ventricular mass. Am J Cardiol. 2004;94:1084–7.
Iacobellis G, Ribaudo MC, Zappaterreno A, et al. Relationship of insulin sensitivity and left ventricular mass in uncomplicated obesity. Obes Res. 2003;11:518–24.
Iacobellis G, Leonetti F, Singh N, et al. Relationship of epicardial adipose tissue with atrial dimensions and diastolic function in morbidly obese subjects. Int J Cardiol. 2007;115:272–3.
Marchington JM, Mattacks CA, Pond CM. Adipose tissue in the mammalian heart and pericardium: structure, foetal development and biochemical properties. Comp Biochem Physiol B. 1989;94:225–32.
Fernandez ML, Volek JS. Guinea pigs: A suitable animal model to study lipoprotein metabolism, atherosclerosis and inflammation. Nutr Metab (Lond). 2006;3:17.
Fernandez ML. Guinea pigs as models for cholesterol and lipoprotein metabolism. J Nutr. 2001;131:10–20.
Tomanek RJ, Searls JC, Lachenbruch PA. Quantitative changes in the capillary bed during developing, peak, and stabilized cardiac hypertrophy in the spontaneously hypertensive rat. Circ Res. 1982;51:295–304.
Loud AV, Beghi C, Olivetti G, et al. Morphometry of right and left ventricular myocardium after strenuous exercise in preconditioned rats. Lab Invest. 1984;51:104–11.
Rösen P, Kiesel U, Reinauer H, et al. Cardiopathy in the spontaneously diabetic (BB) rat. evidence for microangiopathy and autonomic neuropathy in the diabetic heart. In: Nagano M, Dhalla N, editors. The diabetic heart. New York: Raven; 1991. p. 145–57.
Sasson S, Eckel J. Disparate effects of 12-lipoxygenase and 12-hydroxyeicosatetraenoic acid in vascular endothelial and smooth muscle cells and in cardiomyocytes. Arch Physiol Biochem. 2006;112:119–29.
Vahsen S, Rakowski K, Ledwig D, et al. Altered GLUT4 translocation in skeletal muscle of 12/15-lipoxygenase knockout mice. Horm Metab Res. 2006;38:391–6.
Despres JP. Is visceral obesity the cause of the metabolic syndrome? Ann Med. 2006;38:52–63.
Sharma AM, Chetty VT. Obesity, hypertension and insulin resistance. Acta Diabetol. 2005;42:S3–S8.
Kankaanpaa M, Lehto HR, Parkka JP, et al. Myocardial triglyceride content and epicardial fat mass in human obesity: relationship to left ventricular function and serum free fatty acid levels. J Clin Endocrinol Metab. 2006;91:4689–95.
Zhou YT, Grayburn P, Karim A, et al. Lipotoxic heart disease in obese rats: implications for human obesity. Proc Natl Acad Sci U S A. 2000;97:1784–89.
Taegtmeyer H, McNulty P, Young ME. Adaptation and maladaptation of the heart in diabetes: Part I: general concepts. Circulation. 2002;105:1727–33.
Young ME, McNulty P, Taegtmeyer H. Adaptation and maladaptation of the heart in diabetes: Part II: potential mechanisms. Circulation. 2002;105:1861–70.
Iacobellis G, Leonetti F, Singh N, et al. Relationship of epicardial adipose tissue with atrial dimensions and diastolic function in morbidly obese subjects. Int J Cardiol. 2006;15:272–3.
Basso C, Thiene G. Adipositas cordis, fatty infiltration of the right ventricle, and arrhythmogenic right ventricular cardiomyopathy. Just a matter of fat? Cardiovasc Pathol. 2005;14:37–41.
Iacobellis G, Pistilli D, Gucciardo M, et al. Adiponectin expression in human epicardial adipose tissue in vivo is lower in patients with coronary artery disease. Cytokine. 2005;29:251–5.
Iacobellis G, Pellicelli AM, Sharma AM, et al. Relation of subepicardial adipose tissue to carotid intima-media thickness in patients with human immunodeficiency virus. Am J Cardiol. 2007;99:1470–2.
Iacobellis G, Sharma AM, Pellicelli AM, et al. Epicardial adipose tissue is related to carotid intima-media thickness and visceral adiposity in HIV-infected patients with highly active antiretroviral therapy-associated metabolic syndrome. Curr HIV Res. 2007;5:275–9.
Baker AR, Silva NF, Quinn DW, et al. Human epicardial adipose tissue expresses a pathogenic profile of adipocytokines in patients with cardiovascular disease. Cardiovasc Diabetol. 2006;5:1.
Mazurek T, Zhang L, Zalewski A, et al. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation. 2003;108:2460–6.
Dietze D, Koenen M, Rohrig K, et al. Impairment of insulin signaling in human skeletal muscle cells by co-culture with human adipocytes. Diabetes. 2002;51:2369–76.
Gabella G. Cardiovascular system. In: Williams P, editor. Gray’s anatomy 38th edn. Edingburgh: Churchill Livingstone; 1995. p. 1445.
Tansey DK, Aly Z, Sheppard MN. Fat in the right ventricle of the normal heart. Histopathology. 2005;46:98–104.
Hotamisligil GS, Spiegelman BM. Tumor necrosis factor alpha: a key component of the obesity-diabetes link. Diabetes. 1994;43:1271–8.
Scherer PE. Adipose tissue: from lipid storage compartment to endocrine organ. Diabetes. 2006;55:1537–45.
Nguyen-Duy TB, Nichaman MZ, Church TS, et al. Visceral fat and liver fat are independent predictors of metabolic risk factors in men. Am J Physiol Endocrinol Metab. 2003;284:E1065–E1071.
Grimble RF. Genotypic influences on metabolic alterations during inflammation and the nutritional outcome. Nestle Nutr Workshop Ser Clin Perform Programme. 2002;7:1–13.
Grimble RF. Inflammatory status and insulin resistance. Curr Opin Clin Nutr Metab Care. 2002;5:551–9.
Yudkin JS, Eringa E, Stehouwer CD. “Vasocrine” signalling from perivascular fat: a mechanism linking insulin resistance to vascular disease. Lancet. 2005;365:1817–20.
Galili O, Versari D, Sattler KJ, et al. Early experimental obesity is associated with coronary endothelial dysfunction and oxidative stress. Am J Physiol Heart Circ Physiol. 2007;292:H904–H911.
Acknowledgement
We thank D.Herzfeld and H.Müller for their skillful technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Swifka, J., Weiß, J., Addicks, K. et al. Epicardial Fat from Guinea Pig: A Model to Study the Paracrine Network of Interactions between Epicardial Fat and Myocardium?. Cardiovasc Drugs Ther 22, 107–114 (2008). https://doi.org/10.1007/s10557-008-6085-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10557-008-6085-z