Abstract
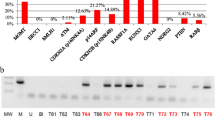
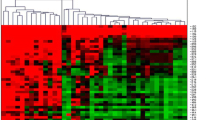
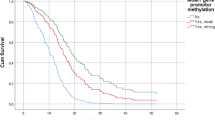
Glioblastoma (GBM) is an aggressive and lethal cancer, accounting for the majority of primary brain tumors in adults. GBMs are characterized by large and small alterations in genes that control cell growth, apoptosis, angiogenesis, and invasion. Epigenetic alterations also affect the expression of cancer genes, either alone or in combination with genetic mechanisms. The current evidence suggests that hypermethylation of promoter CpG islands is a common epigenetic event in a variety of human cancers. A subset of GBMs is also characterized by a locus-specific and genome-wide decrease in DNA methylation. Epigenetic alterations are important in the molecular pathology of GBM. However, there are very limited data about these epigenetic alterations in GBM. Alterations in promoter methylations are important to understand because histone deacetylases are targets for drugs that are in clinical trial for GBMs. The aim of the current study was to investigate whether the promoter hypermethylation of putative tumor suppressor genes was involved in GBM. We examined the methylation status at the promoter regions of GATA6, MGMT, and FHIT using the methylation-specific polymerase chain reaction in 61 primary GBMs. Our results reveal that there is no promoter hypermethylation of FHIT in the examined GBM tissue specimens. In contrast, the promoter hypermethylation of GATA6 and MGMT was detected in 42.8 and 11.11% of GBMs, respectively. The frequency of MGMT promoter hypermethylation was low in the group of patients we evaluated. In conclusion, our study demonstrates that promoter hypermethylation of MGMT is a common event in GBMs, whereas GATA6 is epigenetically affected in GBMs. Furthermore, inactivation of FHIT by epigenetic mechanisms in GBM may not be associated with brain tumorigenesis.


Similar content being viewed by others
Abbreviations
- GBM:
-
Glioblastoma
- MGMT:
-
O6-methylguanine-DNA methyltransferase
- FHIT:
-
Fragile histidine triad
- IHC:
-
Immunohistochemistry
- MSP:
-
Methylation-specific polymerase chain reaction
- WHO:
-
World Health Organization
- LOH:
-
Loss of heterozygosity
References
Ang C, Guiot MC, Ramanakumar AV, Roberge D, Kavan P (2010) Clinical significance of molecular biomarkers in glioblastoma. Can J Neurol Sci 37(5):625–630
Barker FG, Prados MD, Chang SM et al (1996) Radiation response and survival time in patients with glioblastoma multiforme. J Neurosurg 84:442–448
Batchelor TT, Betensky RA, Esposito JM, Pham LD, Dorfman MV, Piscatelli N, Jhung S, Rhee D, Louis DN (2004) Age-dependent prognostic effects of genetic alterations in glioblastoma. Clin Cancer Res 10:228–233
Baylin SB, Herman JG (2000) DNA hypermethylation in tumorigenesis: epigenetics joins genetics. Trends Genet 16:168–174
Belinsky SA (2004) Gene-promoter hypermethylation as a biomarker in lung cancer. Nat Rev Cancer 4:707–717
Cecener G, Tunca B, Egeli U, Bekar A, Guler G, Tolunay S, Aksoy K (2010) FHIT gene sequence variants and reduced Fhit protein expression in glioblastoma multiforme. Cell Mol Neurobiol 30:301–307
Chang Q, Pang JC, Li KK, Poon WS, Zhou L, Ng HK (2005) Promoter hypermethylation profile of RASSF1A, FHIT, and sFRP1 in intracranial primitive neuroectodermal tumors. Hum Pathol 36:1265–1272
Collins VP (2004) Brain tumours: classification and genes. J Neurol Neurosurg Psychiatry 75:2–11
Costello JF, Futscher BW, Kroes RA, Pieper RO (1994a) Methylation-related chromatin structure is associated with exclusion of transcription factors from and suppressed expression of the O-6-Methylguanine DNAmethyltransferase gene in human glioma cell lines. Mol Cell Biol 14:6515–6521
Costello JF, Futscher BW, Tano K, Graunke DM, Pieper RO (1994b) Gradedmethylation in the promoter and body of the O6-methylguanine DNA methyltransferase (MGMT) gene correlates with MGMT expression in human glioma cells. J Biol Chem 269:17228–17237
Curran WJ Jr, Scott CB, Horton J et al (1993) Recursive partitioning analysis of prognostic factors in three radiation therapy oncology group malignant glioma trials. J Natl Cancer Inst 85:704–710
Dong SM, Sun DI, Benoit NE, Kuzmin I, Lerman MI, Sidransky D (2003) Epigenetic inactivation of RASSF1A in head and neck cancer. Clin Cancer Res 9:3635–3640
Donson AM, Addo-Yobo SO, Handler MH, Gore L, Foreman NK (2007) MGMT promoter methylation correlates with survival benefit and sensitivity to temozolomide in pediatric glioblastoma. Pediatr Blood Cancer 48:403–407
Ehrlich M (2003) Expression of various genes is controlled by DNA methylation during mammalian development. J Cell Biochem 88:899–910
Esteller M (2006) Epigenetics provides a new generation of oncogenes and tumour-suppressor genes. Br J Cancer 94:179–183
Esteller M, Hamilton SR, Burger PC, Baylin SB, Herman JG (1999) Inactivation of the DNA repair gene O6-methylguanine-DNA methyltransferase by promoter hypermethylation is a common event in primary human neoplasia. Cancer Res 59:793–797
Everhard S, Kaloshi G, Criniere E et al (2006) MGMT methylation: a marker of response to temozolomide in low-grade gliomas. Ann Neurol 60:740–743
Fraga MF, Esteller M (2005) Towards the human cancer epigenome: a first draft of histone modifications. Cell Cycle 4:1377–1381
Gerson SL (2004) MGMT: its role in cancer aetiology and cancer therapeutics. Nat Rev Canc 4:296–307
Guo M, Akiyama Y, House MG et al (2004) Hypermethylation of the GATA genes in lung cancer. Clin Cancer Res 10:7917–7924
Hegi ME, Diserens AC, Gorlia T et al (2005) MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med 352:997–1003
Herman JG, Baylin SB (2003) Gene silencing in cancer in association with promoter hypermethylation. N Engl J Med 349:2042–2054
Hill C, Hunter SB, Brat DJ (2003) Genetic markers in glioblastoma: prognostic significance and future therapeutic implications. Adv Anat Pathol 10:212–217
Ichimura K, Ohgaki H, Kleihues P, Collins VP (2004) Molecular pathogenesis of astrocytic tumours. J Neurooncol 70:137–160
Jansen M, de Witt Hamer PC, Witmer AN, Troost D, van Noorden CJ (2004) Current perspectives on antiangiogenesis strategies in the treatment of malignant gliomas. Brain Res 45:143–163
Kamnasaran D, Qian B, Hawkins C, Stanford WL, Guha A (2007) GATA6 is an astrocytoma tumor suppressor gene identified by gene trapping of mouse glioma model. Proc Natl Acad Sci USA 104:8053–8058
Kleihues P, Burger PC, Scheithauer BW (1993) The new WHO classification of brain tumours. Brain Pathol 3:255–268
Knudson AG (2000) Chasing the cancer demon. Annu Rev Genet 34:1–19
Martinez R, Martin-Subero JI, Rohde V, Kirsch M, Alaminos M, Fernandez AF, Ropero S, Schackert G, Esteller M (2009) A microarray-based DNA methylation study of glioblastoma multiforme. Epigenetics 4:255–264
McLendon R, Friedman A, Bigner D et al (2008) Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature 455:1061–1068
Michalowski MB, de Fraipont F, Michelland S, Entz-Werle N, Grill J, Pasquier B, Favrot MC, Plantaz D (2006) Methylation of RASSF1A and TRAIL pathway-related genes is frequent in childhood intracranial ependymomas and benign choroid plexus papilloma. Cancer Genet Cytogenet 166:74–81
Myohanen SK, Baylin SB, Herman JG (1998) Hypermethylation can selectively silence individual p16ink4A alleles in neoplasia. Cancer Res 58:591–593
Ogishima T, Shiina H, Breault JE et al (2005) Promoter CpG hypomethylation and transcription factor EGR1 hyperactivate heparanase expression in bladder cancer. Oncogene 24:6765–6772
Ohgaki H, Kleihues P (2005) Population-based studies on incidence, survival rates, and genetic alterations in astrocytic and oligodendroglial gliomas. J Neuropathol Exp Neurol 64:479–489
Ohgaki H, Dessen P, Jourde B et al (2004) Genetic pathways to glioblastoma: a population-based study. Cancer Res 64:6892–6899
Pakneshan P, Xing RH, Rabbani SA (2003) Methylation status of uPA promoter as a molecular mechanism regulating prostate cancer invasion and growth in vitro and in vivo. FASEB J 17:1081–1088
Perry AS, Foley R, Woodson K, Lawler M (2006) The emerging roles of DNA methylation in the clinical management of prostate cancer. Endocr Relat Cancer 13:357–377
Riemenschneider MJ, Hegi ME, Reifenberger G (2010) MGMT promoter methylation in malignant gliomas. Target Oncol 5:161–165
Tamada H, Kitazawa R, Gohji K, Kitazawa S (2001) Epigenetic regulation of human bone morphogenetic protein 6 gene expression in prostate cancer. J Bone Miner Res 16:487–496
Tunca B, Bekar A, Cecener G, Egeli U, Vatan O, Tolunay S, Kocaeli H, Aksoy K (2007) Impact of novel PTEN mutations in Turkish patients with glioblastoma multiforme. J Neurooncol 82:263–269
Yang Q, Shan L, Yoshimura G, Nakamura M, Nakamura Y, Suzuma T, Umemura T, Mori I, Sakurai T, Kakudo K (2002) 5-aza-20-deoxycytidine induces retinoic acid receptor beta 2 demethylation, cell cycle arrest and growth inhibition in breast carcinoma cells. Anticancer Res 22:2753–2756
Zheng S, Ma X, Zhang L, Gunn L, Smith MT, Wiemels JL, Leung K, Buffler PA, Wiencke JK (2004) Hypermethylation of the 5′ CpG island of the FHIT gene is associated with hyperdiploid and translocation-negative subtypes of pediatric leukemia. Cancer Res 646:2000–2006
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cecener, G., Tunca, B., Egeli, U. et al. The Promoter Hypermethylation Status of GATA6, MGMT, and FHIT in Glioblastoma. Cell Mol Neurobiol 32, 237–244 (2012). https://doi.org/10.1007/s10571-011-9753-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-011-9753-7