Abstract
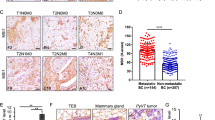
Mena, an actin regulatory protein, functions at the convergence of motility pathways that drive breast cancer cell invasion and migration in vivo. The tumor microenvironment spontaneously induces both increased expression of the Mena invasive (MenaINV) and decreased expression of Mena11a isoforms in invasive and migratory tumor cells. Tumor cells with this Mena expression pattern participate with macrophages in migration and intravasation in mouse mammary tumors in vivo. Consistent with these findings, anatomical sites containing tumor cells with high levels of Mena expression associated with perivascular macrophages were identified in human invasive ductal breast carcinomas and called TMEM. The number of TMEM sites positively correlated with the development of distant metastasis in humans. Here we demonstrate that mouse mammary tumors generated from EGFP-MenaINV expressing tumor cells are significantly less cohesive and have discontinuous cell–cell contacts compared to Mena11a xenografts. Using the mouse PyMT model we show that metastatic mammary tumors express 8.7 fold more total Mena and 7.5 fold more MenaINV mRNA than early non-metastatic ones. Furthermore, MenaINV expression in fine needle aspiration biopsy (FNA) samples of human invasive ductal carcinomas correlate with TMEM score while Mena11a does not. These results suggest that MenaINV is the isoform associated with breast cancer cell discohesion, invasion and intravasation in mice and in humans. They also imply that MenaINV expression and TMEM score measure related aspects of a common tumor cell dissemination mechanism and provide new insight into metastatic risk.







Similar content being viewed by others
Abbreviations
- EGF:
-
Epidermal growth factor
- EGFP:
-
Enhanced green fluorescent protein
- FACs:
-
Fluorescence activated cell sorting
- FFPE:
-
Formalin-fixed paraffin-embedded
- FNA:
-
Fine needle aspiration
- IHC:
-
Immunohistochemistry
- IF:
-
Immunofluorescence
- IVI:
-
Intravital imaging
- qRT-PCR:
-
Quantitative real time polymerase chain reaction
- SCID:
-
Severe combined immunodeficiency
- TMEM:
-
Tumor microenvironment for metastasis
- APTC:
-
Average primary tumor cells
References
Sahai E et al (2005) Simultaneous imaging of GFP, CFP and collagen in tumors in vivo using multiphoton microscopy. BMC Biotechnol 5:14
Condeelis J, Singer RH, Segall JE (2005) The great escape: when cancer cells hijack the genes for chemotaxis and motility. Annu Rev Cell Dev Biol 21:695–718
Wang W et al (2004) Identification and testing of a gene expression signature of invasive carcinoma cells within primary mammary tumors. Cancer Res 64(23):8585–8594
Heimann R et al (2000) Separating favorable from unfavorable prognostic markers in breast cancer: the role of E-cadherin. Cancer Res 60(2):298–304
Weigelt B, Peterse JL, van ‘t Veer LJ (2005) Breast cancer metastasis: markers and models. Nat Rev Cancer 5(8):591–602
Di Modugno F et al (2007) Molecular cloning of hMena (ENAH) and its splice variant hMena+11a: epidermal growth factor increases their expression and stimulates hMena+11a phosphorylation in breast cancer cell lines. Cancer Res 67(6):2657–2665
Gertler F, Condeelis J (2010) Metastasis: tumor cells becoming MENAcing. Trends Cell Biol 21(2):81–90
Goswami S et al (2009) Identification of invasion specific splice variants of the cytoskeletal protein Mena present in mammary tumor cells during invasion in vivo. Clin Exp Metastasis 26(2):153–159
Robinson BD et al (2009) Tumor microenvironment of metastasis in human breast carcinoma: a potential prognostic marker linked to hematogenous dissemination. Clin Cancer Res 15(7):2433–2441
Di Modugno F et al (2006) The cytoskeleton regulatory protein hMena (ENAH) is overexpressed in human benign breast lesions with high risk of transformation and human epidermal growth factor receptor-2-positive/hormonal receptor-negative tumors. Clin Cancer Res 12(5):1470–1478
Gurzu S et al (2008) The expression of cytoskeleton regulatory protein Mena in colorectal lesions. Rom J Morphol Embryol 49(3):345–349
Gurzu S et al (2009) The immunohistochemical aspects of protein Mena in cervical lesions. Rom J Morphol Embryol 50(2):213–216
Gertler FB et al (1996) Mena, a relative of VASP and Drosophila Enabled, is implicated in the control of microfilament dynamics. Cell 87(2):227–239
Bear JE et al (2000) Negative regulation of fibroblast motility by Ena/VASP proteins. Cell 101(7):717–728
Drees F, Gertler FB (2008) Ena/VASP: proteins at the tip of the nervous system. Curr Opin Neurobiol 18(1):53–59
Neel NF et al (2009) VASP is a CXCR2-interacting protein that regulates CXCR2-mediated polarization and chemotaxis. J Cell Sci 122(Pt 11):1882–1894
Philippar U et al (2008) A Mena invasion isoform potentiates EGF-induced carcinoma cell invasion and metastasis. Dev Cell 15(6):813–828
Urbanelli L et al (2006) Characterization of human Enah gene. Biochim Biophys Acta 1759(1–2):99–107
Welch DR, Neri A, Nicolson GL (1983) Comparison of ‘spontaneous’ and ‘experimental’ metastasis using rat 13762 mammary adenocarcinoma metastatic cell clones. Invasion Metastasis 3(2):65–80
Neri A et al (1982) Development and biologic properties of malignant cell sublines and clones of a spontaneously metastasizing rat mammary adenocarcinoma. J Natl Cancer Inst 68(3):507–517
Roussos ET, Balsamo M, Alford SK, Wyckoff JB, Gligorijevic B, Wang Y, Pozzuto M, Stobezki R, Goswami S, Segall JE, Lauffenburger DA, Bresnick AR, Gertler FB, Condeelis JS (2011) Mena invasive (MenaINV) promotes multicellular streaming motility and transendothelial migration in a mouse model of breast cancer. J Cell Sci. doi:10.1242/JCS086231
Wyckoff J et al (2004) A paracrine loop between tumor cells and macrophages is required for tumor cell migration in mammary tumors. Cancer Res 64(19):7022–7029
Wang W et al (2002) Single cell behavior in metastatic primary mammary tumors correlated with gene expression patterns revealed by molecular profiling. Cancer Res 62(21):6278–6288
Wyckoff J et al (2010) High resolution multi-photon imaging of tumors in vivo. In: Goldman RD, Swedlow JR, Spector DL (eds) Live cell imaging; a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, pp 441–462
Zipfel WR, Williams RM, Webb WW (2003) Nonlinear magic: multiphoton microscopy in the biosciences. Nat Biotechnol 21(11):1369–1377
Gupta PK, Baloch ZW (2002) Intraoperative and on-site cytopathology consultation: utilization, limitations, and value. Semin Diagn Pathol 19(4):227–236
Mouneimne G et al (2004) Phospholipase C and cofilin are required for carcinoma cell directionality in response to EGF stimulation. J Cell Biol 166(5):697–708
Loureiro JJ et al (2002) Critical roles of phosphorylation and actin binding motifs, but not the central proline-rich region, for Ena/vasodilator-stimulated phosphoprotein (VASP) function during cell migration. Mol Biol Cell 13(7):2533–2546
Lebrand C et al (2004) Critical role of Ena/VASP proteins for filopodia formation in neurons and in function downstream of netrin-1. Neuron 42(1):37–49
Pino MS et al (2008) Human Mena+11a isoform serves as a marker of epithelial phenotype and sensitivity to epidermal growth factor receptor inhibition in human pancreatic cancer cell lines. Clin Cancer Res 14(15):4943–4950
Lin EY et al (2003) Progression to malignancy in the polyoma middle T oncoprotein mouse breast cancer model provides a reliable model for human diseases. Am J Pathol 163(5):2113–2126
Symmans WF et al (2003) Total RNA yield and microarray gene expression profiles from fine-needle aspiration biopsy and core-needle biopsy samples of breast carcinoma. Cancer 97(12):2960–2971
Di Modugno F et al (2004) Human Mena protein, a serex-defined antigen overexpressed in breast cancer eliciting both humoral and CD8+ T-cell immune response. Int J Cancer 109(6):909–918
Giampieri S et al (2009) Localized and reversible TGFbeta signalling switches breast cancer cells from cohesive to single cell motility. Nat Cell Biol 11(11):1287–1296
Zeineldin R et al (2006) Mesenchymal transformation in epithelial ovarian tumor cells expressing epidermal growth factor receptor variant III. Mol Carcinog 45(11):851–860
Guy CT, Cardiff RD, Muller WJ (1992) Induction of mammary tumors by expression of polyomavirus middle T oncogene: a transgenic mouse model for metastatic disease. Mol Cell Biol 12(3):954–961
Lin EY et al (2001) Colony-stimulating factor 1 promotes progression of mammary tumors to malignancy. J Exp Med 193(6):727–740
Wyckoff JB et al (2000) A critical step in metastasis: in vivo analysis of intravasation at the primary tumor. Cancer Res 60(9):2504–2511
Wyckoff JB et al (2007) Direct visualization of macrophage-assisted tumor cell intravasation in mammary tumors. Cancer Res 67(6):2649–2656
Wang W et al (2005) Tumor cells caught in the act of invading: their strategy for enhanced cell motility. Trends Cell Biol 15(3):138–145
Wang W et al (2007) Coordinated regulation of pathways for enhanced cell motility and chemotaxis is conserved in rat and mouse mammary tumors. Cancer Res 67(8):3505–3511
Acknowledgments
We would like to thank Drs. Jeffery Segall, Diane Cox, Antonia Patsialou, and Daqian Sun for stimulating discussion and helpful suggestions. We also thank Drs. Sasis Sirikanjanapong, Jason Moss, and Zhong, as well as research associate Mrs. Felicia Juliano for assistance with FNA biopsy procedures, Dr. Jaya Sunkara for assistance with E-cadherin staining, and Dr. Olena Dorokhova for help with RNA extraction and cDNA synthesis. Many thanks to David Entenberg, and Jenny Tadros for their technical support, Einstein histopathology, Analytical Imaging Facilities, and Koch Institute for Microscopy, Histology and Flow Cytometry sorting core facilities for their services. Grant support provided by CA100324 (SG, YW), CA113395 (MHO), CA150344 (ETR), AECC9526-5267 (MHO, SG), Ludwig Fund postdoctoral fellowship (MB), GM58801 and funds from the Ludwig center at MIT (FBG), ICBP grant U54 CA112967 (FBG).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Roussos, E.T., Goswami, S., Balsamo, M. et al. Mena invasive (MenaINV) and Mena11a isoforms play distinct roles in breast cancer cell cohesion and association with TMEM. Clin Exp Metastasis 28, 515–527 (2011). https://doi.org/10.1007/s10585-011-9388-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10585-011-9388-6