Abstract
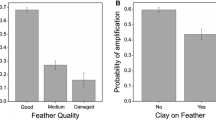
The use of non-destructive sampling methods to collect genetic material from wildlife allows researchers to minimize disturbance. Most avian studies employ capturing and handling of young and parents to draw blood for DNA analysis. In some cases adult female birds are difficult to catch, so maternal genotyping has required collection of contour feathers from nests, or destructive sampling of eggs. Many species do not leave contour feathers in the nest, and destructive sampling has been unreliable due to contamination with embryonic DNA. Alternative field sampling techniques for collection of maternal DNA from birds are therefore desirable. Here we demonstrate that avian maternal DNA can be isolated in a non-invasive and non-destructive way from the external surface of eggs. We used cotton swabs to collect maternal DNA from the external shells of herring gull (Larus argentatus) and Caspian tern (Sterna caspia) eggs. DNA was then amplified by the polymerase chain reaction (PCR) for microsatellite genotyping. We verified that the DNA samples were maternal by comparing microsatellite profiles to those obtained from adults and chicks from the same nests. In 100% of Caspian tern (n=16) and herring gull families (n=12), the egg swabs that amplified matched the maternal microsatellite genotype. In a screening of many nests of both species, we successfully amplified microsatellite markers from 101/115 (88%) egg swabs. Swabs from eggs with blood stains on the shell were more likely to amplify successfully than those from clean eggs. The advantages of this new method include increased parentage assignment/exclusion power, and increased availability of maternal DNA for genotyping of species that do not deposit contour feathers in nests.
Similar content being viewed by others
References
Avise JC (2004) Molecular Markers, Natural History, and Evolution, 2nd edition. Sinauer.
Bety J, Gauthier G (2001) Effects of nest visits on predator activity and predation rate in a Greater Snow Goose colony. J. Field Ornithol. 72, 573–586.
Cariello MO, Schwabl HG, Lee RW, Macedo RH (2002) Individual female clutch identification through yolk protein electrophoresis in the communally-breeding guira cuckoo (Guira guira). Mol. Ecol., 11, 2417–2424.
Cotter RC, Gratto CJ (1995) Effects of nest and brood visits and radio transmitters on Rock Ptarmigan. J. Wild. Manage., 1, 93-98.
Criscuolo F (2001) Does blood sampling during incubation induce nest desertion in the female Common Eider Somateria mollissima? Marine Ornithol. 29, 47–50.
Emlen ST, Wrege PH (2004) Size dimorphism, intrasexual competition, and sexual selection in wattled jacana (Jacana jacana), a sex-role-reversed shorebird in Panama. Auk, 121, 391–403.
Flagstad Ø, Røed K, Stacy JE, Jakobsen KS (1999) Reliable non-invasive genotyping based on excremental PCR of nuclear DNA purified with a magnetic bead protocol. Mol. Ecol., 8, 879–883.
Gibbs HL, Sorenson MD, Marchetti K, Brooke MD, Davies NB, Nakamura H (2000) Genetic evidence for female host-specific races of the common cuckoo. Nature, 407, 183–186.
Götmark F (1992) The effects of investigator disturbance on nesting birds. Curr. Ornithol., 9, 63–104.
Gregory SM, Quinn JS Microsatellite isolation from four avian species comparing two isolation techniques. Mol. Ecol. Notes, in press.
Griffiths R, Double MC, Orr K, Dawson RJG (1998) A DNA test to sex most birds. Mol. Ecol., 7, 1071–1075.
Jarne P, Ladoga PJL (1996) Microsatellites, from molecules to populations and back. Trends Ecol. Evol., 11, 424–429.
Komeda, S (1983) Nest attendance of parent birds in the painted snipe (Rostratula benghalensis). Auk, 100, 48–55.
Macedo RHF, Cariello MO, Pacheco AM, Schwabl HG (2004) Significance of social parameters on differential nutrient investment in guira cuckoo, Guira guira, eggs. Anim. Behav., 68, 485–494.
MayerGross H, Crick HQP, Greewood JJD (1997) The effect of observers visiting the nests of passerines: An experimental study. Bird Study, 44, 53–65.
Parsons J (1976) Factors determining number and size of eggs laid by herring gulls. Condor, 78, 481–497.
Pearce JM, Fields RL, Scribner, KT (1997) Nest materials as a source of genetic data for avian ecological studies. J. Field Ornithol., 68, 471–481.
Queller DC, Strassmann JE, Hughes CR (1993) Microsatellites and kinship. Trends Ecol. Evol., 8, 285–288.
Quinn JS, Morris RD (1986) Intraclutch egg-weight apportionment and chick survival in Caspian terns. Can. J. Zool., 64, 2116–2122.
Quinn JS, Morris RD, Blokpoel H, Weseloh DV, Ewins P (1996) Design and management of bird nesting habitat: Tactics for conserving colonial waterbird biodiversity on artificial islands in Hamilton Harbour, Ontario. Can. J. Fish. Aquat. Sci., 53, 45–57.
Quinn JS, Sirdevan J (1998) Experimental measurement of nesting substrate preference in Caspian Terns, Sterna caspia and the successful colonisation of human-constructed islands. Biol. Conserv., 85, 63–68.
Quinn JS, Startek-Foote JM (2000) Smooth-billed ani (Crotophaga ani). In: The Birds of North America (eds. Poole A, Gill F), Vol. 539, pp. 1–16. The Birds of North America, Inc., Philadelphia, PA
Safina C. and Buger J (1983). Effect of human disturbance on reproductive success in the black skimmer. Condor, 85, 164–171.
Singer VL, Jones LJ, Yue ST, Haugland RP (1997) Characterization of PicoGreen reagent and development of a fluorescence-based solution assay for double-stranded DNA quantitation. Anal. Biochem., 249, 228–238.
Stouffer, PC (1997) Interspecific Aggression in Formicarius Antthrushes? The view from Central Amazonian Brazil. Auk, 114, 780–785.
Strausberger BM, Ashley ME (2001) Eggs yield nuclear DNA from egg-laying female cowbirds, their embryos and offspring. Conserv. Genet., 2, 385–390.
Taberlet P, Waits LP, Luikart G (1999) Non-invasive genetic sampling: Look before you leap. Trends Ecol. Evol., 14, 323–327.
Vehrencamp SL, Quinn JS (2004) Joint laying systems. In: Ecology and Evolution of Cooperative Breeding in Birds (eds. Koening WD, Dickinson JL), pp. 177–278. Cambridge University Press.
Verboven N, Ens BJ, Dechesne, S (2001) Effect of investigator disturbance on nest attendance and egg predation in Eurasian oystercatchers. Auk, 118, 503–508.
Winfree R (1999) Cuckoos, cowbirds and the persistence of brood parasitism. Trends Ecol. Evol., 14, 338–343.
Acknowledgements
This work was supported by a grant from the National Science and Engineering Research council (NSERC) of Canada to JSQ, and by Ontario Graduate Scholarships and McMaster University graduate student scholarships to GS and to CMS. Field work was performed under permit from Environment Canada. We thank the Canadian Wildlife Service and the Canada Centre for Inland Waters for logistical support. Finally, we would like to thank two anonymous reviewers for improving the quality of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schmaltz, G., Somers, C.M., Sharma, P. et al. Non-destructive sampling of maternal DNA from the external shell of bird eggs. Conserv Genet 7, 543–549 (2006). https://doi.org/10.1007/s10592-005-9065-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10592-005-9065-x