Abstract
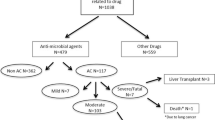
Objective To review all cases of drug-induced liver injury (DILI) requiring hospitalization at a single tertiary care center. Methods Patient records were identified by ICD-9 codes for inpatient visits from November 1998 through March 2006. Results Of a total 83,265 hospital admissions during the study period, 40 were for DILI (0.048%). Thirteen patients had non-acetaminophen DILI (NA-DILI); 27 had acetaminophen-related DILI (A-DILI). In the NA-DILI group, mean age was 59 ± 17.9 years and liver injury was classified as hepatocellular (7), cholestatic (5), or mixed (1). A variety of medications were implicated with antimicrobials being the most common class. Resolution occurred in seven, two died of complications related to hepatotoxicity, one underwent liver transplantation, and the outcome was undetermined in three who were lost to follow-up. In the A-DILI group, mean age was 35 ± 11.0 years. Eighteen involved intentional overdose of acetaminophen; nine were associated with chronic use. The pattern of injury was hepatocellular in all. Resolution occurred in 4 patients, death in 8, and improvement in 15. Conclusions DILI is a rare cause of inpatient admission but is associated with significant mortality. Spontaneous resolution occurs in most patients but return to normal liver function may take months. Antimicrobial agents account for the largest proportion of NA-DILI.
Similar content being viewed by others
Abbreviations
- A-DILI:
-
Acetaminophen drug-induced liver injury
- ALT:
-
Alanine aminotransferase
- AP:
-
Alkaline phosphatase
- AST:
-
Aspartate aminotransferase
- DILI:
-
Drug-induced liver injury
- FHF:
-
Fulminant hepatic failure
- IQR:
-
Interquartile range
- LOS:
-
Length of stay
- NA-DILI:
-
Non-acetaminophen drug-induced liver injury
- ULN:
-
Upper limit of normal
References
Sgro C, Clinard F, Ouazir K, Chanay H, Allard C, Guilleminet C, Lenoir C, Lemoine A, Hillon P (2002) Incidence of drug-induced hepatic injuries: a French population-based study. Hepatology 36:451–455
Ostapowicz G, Fontana RJ, Schiodt FV, Larson A, Davern TJ, Han SHB, McCashland TM, Shakil AO, Hay JE, Hynan L, Crippin JS, Blei AT, Samuel G, Reisch J, Lee WM, the U.S. Acute Liver Failure Study Group (2002) Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med 137:947–954
Bakke OM, Manocchia M, De Abajo F, Kaitin KI, Lasagna L (1995) Drug safety discontinuations in the United Kingdom, the United States, and Spain from 1974 through 1993: a regulatory perspective. Clin Pharmacol Ther 58:108–117
Benichou C (1990) Criteria of drug-induced liver disorders. Report of an international consensus meeting. J Hepatol 11:272–276
Danan G, Benichou C (1999) Causality assessment of adverse reactions to drugs—I. A novel method based on the conclusions of international consensus meetings: application to drug-induced liver injuries. J Clin Epidemiol 46:1323–1330
Bjornsson E, Jerlstad P, Bergqvist A, Olsson R (2005) Fulminant drug-induced hepatic failure leading to death or liver transplantation in Sweden. Scand J Gastroenterol 40:1095–1101
Drug-Induced Liver Injury Network. Available at: http://dilin.dcri.duke.edu/. Accessibility verified November 21, 2006
Ibanez L, Perez E, Vidal X, Laporte JR, the Grup d’Estudi Multicentric d’Hepatotoxicitat Aguda de Barcelona (GEMHAB) (2002) Prospective surveillance of acute serious liver disease unrelated to infectious, obstructive, or metabolic diseases: epidemiological and clinical features, and exposure to drugs. J Hepatol 37:592–600
Andrade RJ, Lucena MI, Fernandez MC, Pelaez G, Pachkoria K, Garcia-Ruiz E, Garcia-Munoz B, Gonzalez-Grande R, Pizarro A, Duran JA, Jimenez M, Rodrigo L, Romero-Gomez M, Navarro JM, Planas R, Costa J, Borras A, Soler A, Salmeron J, Martin-Vivaldi R (2005) Drug-induced liver injury: an analysis of 461 incidences submitted to the Spanish registry over a 10-year period. Gastroenterology 129:512–521
Wei G, Bergqvist A, Broome U (2004) Acute liver failure in Sweden: etiology and prognosis [abstract 48]. Scand J Gastroenterol 240(39 Suppl):36
O’Grady JG, Alexander GJM, Hayllar KM, Williams R (1989) Early indicators of prognosis in fulminant hepatic failure. Gastroenterology 97:439–445
Friis H, Andreasen PB (1992) Drug-induced hepatic injury: an analysis of 1100 cases reported to the Danish committee on adverse drug reactions between 1978 and 1987. J Intern Med 232:133–138
Galan MV, Potts JA, Silverman AL, Gordon SC (2005) The burden of acute nonfulminant drug-induced hepatitis in a United States tertiary referral center. J Clin Gastroenterol 39:64–67
Russo MW, Galanko JA, Shrestha R, Fried MW, Watkins P (2004) Liver transplantation for acute liver failure from drug-induced liver injury in the United States. Liver Transpl 10:1018–1023
Smith IDM, Simpson KJ, Garden OJ, Wigmore SJ (2005) Non-paracetamol drug-induced fulminant hepatic failure among adults in Scotland. Eur J Gastroenterol Hepatol 17:161–167
Aithal PG, Day CP (1999) The natural history of histologically proved drug induced liver disease. Gut 44:731–735
Eisenberg DM, Davis RB, Ettner SL, Appel S, Wilkey S, Van Rompay M, Kessler RC (1998) Trends in alternative medicine use in the United States, 1990–1997. JAMA 280:1569–1575
Larrey D (1997) Hepatotoxicity of herbal remedies. J Hepatol 26(Suppl 1):47–51
Saper RB, Kales SN, Paquin J, Burns MJ, Eisenberg DM, Davis RB, Phillips RS (2004) Heavy metal content of ayurvedic herbal medicine products. JAMA 292:2868–2873
Zimmerman HJ (1999) Hepatotoxicity. The adverse effects of drugs and other chemicals on the liver, 2nd edn. Philadelphia: Lippincott Williams & Wilkins
Hawton K, Simkin K, Deeks K, Cooper J, Johnston A, Waters K, Arundel M, Bernal W, Hudson M, Suri D, Simpson K (2004) UK legislation on analgesic packs: before and after study on long term effect of poisonings. Br Med J 329:1076–1081
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Carey, E.J., Vargas, H.E., Douglas, D.D. et al. Inpatient Admissions for Drug-induced Liver Injury: Results from a Single Center. Dig Dis Sci 53, 1977–1982 (2008). https://doi.org/10.1007/s10620-008-0250-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-008-0250-x