Summary
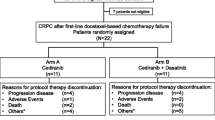
Background Integrins are involved in prostate cancer metastasis by regulating cell adhesion, migration, invasion, motility, angiogenesis and bone metabolism. We evaluated the efficacy of two dose levels of cilengitide in patients (pts) with castrate resistant prostate cancer (CRPC). Methods Chemotherapy-naïve, asymptomatic metastatic CRPC pts were randomized to cilengitide 500 mg or 2,000 mg IV twice weekly using parallel 2-stage design. The primary endpoint was rate of objective clinical progression at 6-months. Secondary endpoints included clinical and PSA response rates, safety and effects of cilengitide treatment on circulating tumor cells (CTCs) and bone remodeling markers. Results Forty-four pts were accrued to first stage (22/arm). Median number of cycles was three in both arms (500 mg arm: 1–8; 2,000 mg arm: 1–15). At 6 months, two pts (9%) on the 500 mg arm and five pts (23%) on the 2,000 mg arm had not progressed. Best objective response was stable disease (SD) in seven pts for 9.9[8.1,20.9] months. There were three grade 3 and no grade 4 toxicities. At 12 weeks, analysis of bone markers did not reveal significant trends. At progression, bone specific alkaline phosphatase and N-telopeptide increased in all pts, less so in pts on the 2,000 mg arm and in pts on both arms who obtained SD at 6 months. CTCs increased over time in both arms. Conclusion Cilengitide was well tolerated with modest clinical effect in favor of the higher dose. The unique trial design including a shift from response rate to objective progression as the endpoint, and not acting on PSA increases was feasible.


Similar content being viewed by others
References
EMD 121974 Cilengitide, cyclo-[Arg-Gly-Asp-DPhe-(NMeVal)]. Investigator’s Brochure
Albelda SM (1993) Role of integrins and other cell adhesion molecules in tumor progression and metastasis. Lab Invest 68:4–17
Allen MV, Smith GJ, Juliano R, Maygarden SJ, Mohler JL (1998) Downregulation of the beta4 integrin subunit in prostatic carcinoma and prostatic intraepithelial neoplasia. Hum Pathol 29:311–318
Assoian RK, Schwartz MA (2001) Coordinate signaling by integrins and receptor tyrosine kinases in the regulation of G1 phase cell-cycle progression. Curr Opin Genet Dev 11:48–53
Brooks PC, Montgomery AM, Rosenfeld M, Reisfeld RA, Hu T, Klier G, Cheresh DA (1994) Integrin alpha v beta 3 antagonists promote tumor regression by inducing apoptosis of angiogenic blood vessels. Cell 79:1157–1164
Brown JE, Cook RJ, Major P, Lipton A, Saad F, Smith M, Lee KA, Zheng M, Hei YJ, Coleman RE (2005) Bone turnover markers as predictors of skeletal complications in prostate cancer, lung cancer, and other solid tumors. J Natl Cancer Inst 97:59–69
Bubley GJ, Carducci M, Dahut W, Dawson N, Daliani D, Eisenberger M, Figg WD, Freidlin B, Halabi S, Hudes G, Hussain M, Kaplan R, Myers C, Oh W, Petrylak DP, Reed E, Roth B, Sartor O, Scher H, Simons J, Sinibaldi V, Small EJ, Smith MR, Trump DL, Wilding G et al (1999) Eligibility and response guidelines for phase II clinical trials in androgen-independent prostate cancer: recommendations from the prostate-specific antigen working group. J Clin Oncol 17:3461–3467
Carducci M, Nelson JB, Saad F, Schulman CC, Dearnaley DP, Sleep DJ, Hulting SM, Isaacson JD, Allen A, Nisen P (2004) Effects of atrasentan on disease progression and biological markers in men with metastatic hormone-refractory prostate cancer: phase 3 study. J Clin Oncol 22:4508
Carducci MA, Padley RJ, Breul J, Vogelzang NJ, Zonnenberg BA, Daliani DD, Schulman CC, Nabulsi AA, Humerickhouse RA, Weinberg MA, Schmitt JL, Nelson JB (2003) Effect of endothelin-A receptor blockade with atrasentan on tumor progression in men with hormone-refractory prostate cancer: a randomized, phase II, placebo-controlled trial. J Clin Oncol 21:679–689
Carducci MA, Saad F, Abrahamsson PA, Dearnaley DP, Schulman CC, North SA, Sleep DJ, Isaacson JD, Nelson JB (2007) A phase 3 randomized controlled trial of the efficacy and safety of atrasentan in men with metastatic hormone-refractory prostate cancer. Cancer 110:1959–1966
Coleman RE, Major P, Lipton A, Brown JE, Lee KA, Smith M, Saad F, Zheng M, Hei YJ, Seaman J, Cook R (2005) Predictive value of bone resorption and formation markers in cancer patients with bone metastases receiving the bisphosphonate zoledronic acid. J Clin Oncol 23:4925–4935
Cooper CR, Chay CH, Pienta KJ (2002) The role of alpha(v)beta(3) in prostate cancer progression. Neoplasia 4:191–194
Cooper CR, Pienta KJ (2000) Cell adhesion and chemotaxis in prostate cancer metastasis to bone: a minireview. Prostate Cancer Prostatic Dis 3:6–12
Danila DC, Heller G, Gignac GA, Gonzalez-Espinoza R, Anand A, Tanaka E, Lilja H, Schwartz L, Larson S, Fleisher M, Scher HI (2007) Circulating tumor cell number and prognosis in progressive castration-resistant prostate cancer. Clin Cancer Res 13:7053–7058
Davis TL, Cress AE, Dalkin BL, Nagle RB (2001) Unique expression pattern of the alpha6beta4 integrin and laminin-5 in human prostate carcinoma. Prostate 46:240–248
de Bono JS, Scher HI, Montgomery RB, Parker C, Miller MC, Tissing H, Doyle GV, Terstappen LW, Pienta KJ, Raghavan D (2008) Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res 14:6302–6309
Duong LT, Lakkakorpi P, Nakamura I, Rodan GA (2000) Integrins and signaling in osteoclast function. Matrix Biol 19:97–105
Eskens FA, Dumez H, Hoekstra R, Perschl A, Brindley C, Bottcher S, Wynendaele W, Drevs J, Verweij J, van Oosterom AT (2003) Phase I and pharmacokinetic study of continuous twice weekly intravenous administration of Cilengitide (EMD 121974), a novel inhibitor of the integrins alphavbeta3 and alphavbeta5 in patients with advanced solid tumours. Eur J Cancer 39:917–926
Fornaro M, Manes T, Languino LR (2001) Integrins and prostate cancer metastases. Cancer Metastasis Rev 20:321–331
Hariharan S, Gustafson D, Holden S, McConkey D, Davis D, Morrow M, Basche M, Gore L, Zang C, O’Bryant CL, Baron A, Gallemann D, Colevas D, Eckhardt SG (2007) Assessment of the biological and pharmacological effects of the alpha nu beta3 and alpha nu beta5 integrin receptor antagonist, cilengitide (EMD 121974), in patients with advanced solid tumors. Ann Oncol 18:1400–1407
Holly SP, Larson MK, Parise LV (2000) Multiple roles of integrins in cell motility. Exp Cell Res 261:69–74
Hood JD, Cheresh DA (2002) Role of integrins in cell invasion and migration. Nat Rev Cancer 2:91–100
Hughes DE, Salter DM, Dedhar S, Simpson R (1993) Integrin expression in human bone. J Bone Miner Res 8:527–533
Keller ET, Brown J (2004) Prostate cancer bone metastases promote both osteolytic and osteoblastic activity. J Cell Biochem 91:718–729
Knox JD, Cress AE, Clark V, Manriquez L, Affinito KS, Dalkin BL, Nagle RB (1994) Differential expression of extracellular matrix molecules and the alpha 6-integrins in the normal and neoplastic prostate. Am J Pathol 145:167–174
Lara PN Jr, Stadler WM, Longmate J, Quinn DI, Wexler J, Van Loan M, Twardowski P, Gumerlock PH, Vogelzang NJ, Vokes EE, Lenz HJ, Doroshow JH, Gandara DR (2006) A randomized phase II trial of the matrix metalloproteinase inhibitor BMS-275291 in hormone-refractory prostate cancer patients with bone metastases. Clin Cancer Res 12:1556–1563
Lipton A, Cook R, Saad F, Major P, Garnero P, Terpos E, Brown JE, Coleman RE (2008) Normalization of bone markers is associated with improved survival in patients with bone metastases from solid tumors and elevated bone resorption receiving zoledronic acid. Cancer 113:193–201
MacDonald TJ, Stewart CF, Kocak M, Goldman S, Ellenbogen RG, Phillips P, Lafond D, Poussaint TY, Kieran MW, Boyett JM, Kun LE (2008) Phase I clinical trial of cilengitide in children with refractory brain tumors: pediatric brain tumor consortium study PBTC-012. J Clin Oncol 26:919–924
Moreno J, DeBono JS, Shaffer D, Montgomery B, Miller MC, Tissing H, Doyle G, Terstappen LW, Pienta KJ, Raghavan D (2007) Multi-center study evaluating circulating tumor cells (CTCs) as a surrogate for survival in men treated for castration refractory prostate cancer (CRPC). J Clin Oncol 25: abstract 5016
Moreno JG, Miller MC, Gross S, Allard WJ, Gomella LG, Terstappen LW (2005) Circulating tumor cells predict survival in patients with metastatic prostate cancer. Urology 65:713–718
Murant SJ, Handley J, Stower M, Reid N, Cussenot O, Maitland NJ (1997) Co-ordinated changes in expression of cell adhesion molecules in prostate cancer. Eur J Cancer 33:263–271
Nabors LB, Mikkelsen T, Rosenfeld SS, Hochberg F, Akella NS, Fisher JD, Cloud GA, Zhang Y, Carson K, Wittemer SM, Colevas AD, Grossman SA (2007) Phase I and correlative biology study of cilengitide in patients with recurrent malignant glioma. J Clin Oncol 25:1651–1657
Nagle RB, Knox JD, Wolf C, Bowden GT, Cress AE (1994) Adhesion molecules, extracellular matrix, and proteases in prostate carcinoma. J Cell Biochem Suppl 19:232–237
Nakamura I, Pilkington MF, Lakkakorpi PT, Lipfert L, Sims SM, Dixon SJ, Rodan GA, Duong LT (1999) Role of alpha(v)beta(3) integrin in osteoclast migration and formation of the sealing zone. J Cell Sci 112(Pt 22):3985–3993
Nemeth JA, Cher ML, Zhou Z, Mullins C, Bhagat S, Trikha M (2003) Inhibition of alpha(v)beta3 integrin reduces angiogenesis, bone turnover, and tumor cell proliferation in experimental prostate cancer bone metastases. Clin Exp Metastasis 20:413–420
Pidgeon GP, Tang K, Cai YL, Piasentin E, Honn KV (2003) Overexpression of platelet-type 12-lipoxygenase promotes tumor cell survival by enhancing alpha(v)beta(3) and alpha(v)beta(5) integrin expression. Cancer Res 63:4258–4267
Reardon D, Fink K, Nabors B, Cloughesy T, Plotkin S, Schiff D, Raizer J, Krueger S, Picard M, Mikkelsen T (2007) Phase IIa trial of cilengitide (EMD121974) single-agent therapy in patients (pts) with recurrent glioblastoma (GBM): EMD 121974-009. J Clin Oncol 25: abstract 2002
Rodan SB, Rodan GA (1997) Integrin function in osteoclasts. J Endocrinol 154(Suppl):S47–S56
Rowand JL, Martin G, Doyle GV, Miller MC, Pierce MS, Connelly MC, Rao C, Terstappen LW (2007) Endothelial cells in peripheral blood of healthy subjects and patients with metastatic carcinomas. Cytometry A 71:105–113
Ruoslahti E, Reed JC (1994) Anchorage dependence, integrins, and apoptosis. Cell 77:477–478
Saad F, Gleason DM, Murray R, Tchekmedyian S, Venner P, Lacombe L, Chin JL, Vinholes JJ, Goas JA, Chen B (2002) A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J Natl Cancer Inst 94:1458–1468
Scher HI, Halabi S, Tannock I, Morris M, Sternberg CN, Carducci MA, Eisenberger MA, Higano C, Bubley GJ, Dreicer R, Petrylak D, Kantoff P, Basch E, Kelly WK, Figg WD, Small EJ, Beer TM, Wilding G, Martin A, Hussain M (2008) Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the prostate cancer clinical trials working group. J Clin Oncol 26:1148–1159
Schmelz M, Cress AE, Scott KM, Burger F, Cui H, Sallam K, McDaniel KM, Dalkin BL, Nagle RB (2002) Different phenotypes in human prostate cancer: alpha6 or alpha3 integrin in cell-extracellular adhesion sites. Neoplasia 4:243–254
Shah RB, Mehra R, Chinnaiyan AM, Shen R, Ghosh D, Zhou M, Macvicar GR, Varambally S, Harwood J, Bismar TA, Kim R, Rubin MA, Pienta KJ (2004) Androgen-independent prostate cancer is a heterogeneous group of diseases: lessons from a rapid autopsy program. Cancer Res 64:9209–9216
Simon R, Wittes RE, Ellenberg SS (1985) Randomized phase II clinical trials. Cancer Treat Rep 69:1375–1381
Slack-Davis JK, Parsons JT (2004) Emerging views of integrin signaling: implications for prostate cancer. J Cell Biochem 91:41–46
Stewart DA, Cooper CR, Sikes RA (2004) Changes in extracellular matrix (ECM) and ECM-associated proteins in the metastatic progression of prostate cancer. Reprod Biol Endocrinol 2:2
Tantivejkul K, Kalikin LM, Pienta KJ (2004) Dynamic process of prostate cancer metastasis to bone. J Cell Biochem 91:706–717
Vogelzang N, Nelson J, Schulman C, Dearnaley D, Saad F, Sleep D, Isaacson J, Carducci M (2005) Meta-analysis of clinical trials of atrasentan 10 mg in metastatic hormone-refractory prostate cancer. J Clin Oncol 23:4563
Zheng DQ, Woodard AS, Fornaro M, Tallini G, Languino LR (1999) Prostatic carcinoma cell migration via alpha(v)beta3 integrin is modulated by a focal adhesion kinase pathway. Cancer Res 59:1655–1664
Acknowledgements
CTEP, Prostate SPORE Grant P50 CA069568-09, Merck KGaA, PC051382, PC051375, PCF N008367, Immunicon Corporation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Authors of the DOD Prostate Cancer Clinical Trial Consortium also included Kathleen A. Cooney, Kenneth J. Pienta, MD, Paul Mathew, MD, June Escara-Wilke, MS, Mahmoud Al-Hawary, MD, Evan Keller, DVM, PhD, and Gerald Doyle DDS
This study was presented in part at the 2007 and 2008 American Society of Clinical Oncology Annual Meeting and the 2007 Department of Defense Innovative Minds in Prostate Cancer Treatment (IMPACT 2007) Meeting.
An erratum to this article can be found at http://dx.doi.org/10.1007/s10637-010-9502-7
Rights and permissions
About this article
Cite this article
Bradley, D.A., Daignault, S., Ryan, C.J. et al. Cilengitide (EMD 121974, NSC 707544) in asymptomatic metastatic castration resistant prostate cancer patients: a randomized phase II trial by the prostate cancer clinical trials consortium. Invest New Drugs 29, 1432–1440 (2011). https://doi.org/10.1007/s10637-010-9420-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-010-9420-8