Abstract
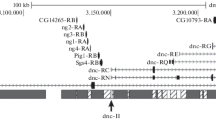
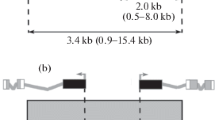
The 3A and 60E regions of Drosophila melanogaster polytene chromosomes containing inserted copies of the P{lArB} transposon have been subjected to an electron microscopic (EM) analysis. We show that both inserts led to formation of new bands within the interband regions 3A4/A6 and 60E8-9/E10. This allowed us to clone DNA of these interbands. Their sequences, as well as those of DNA from other four interbands described earlier, have been analyzed. We have found that, with the exception of 60E8-9/E10 interband, all other five regions under study corresponded to 5' or 3' ends of genes. We have further obtained the evidence for 60E8-9/E10 interband to harbor the 'housekeeping' RpL19 gene, which is transcribed in many tissues, including salivary glands. Based upon the genetic heterogeneity of the interbands observed a revised model of polytene chromosome organization is discussed.
Similar content being viewed by others
References
Adams, M. D., S. E. Celniker, R. A. Holt, C. A. Evans et al., 2000. The genome sequence of Drosophila melanogaster. Science 287: 2185–2195.
Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Zh. Zhang, W. Miller & D. J. Lipman, 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucl. Acids Res. 25: 3389–3402.
Beermann, W., 1972. Chromomeres and genes, pp.1–33 in Results and Problems in Cell Differentiation, Vol. 4, edited by W. Beermann. Springer, Berlin, Heidelberg, New York.
Bonner, J. J., C. Parks, J. Parker-Thornburg, M. A. Mortin & H. R. B. Pelham, 1984. The use of promoter fusions in Drosophila genetics: isolation of mutations a. ecting the heat shock response. Cell 37: 979–991.
Bridges, C. B., 1938. A revised map of the salivary gland X-chromosome of Drosophila melanogaster. J. Hered. 29: 11–13.
Carrera, P., S. Abrell, B. Kerber, U. Walldorf, A. Preiss, M. Hoch & H. Jackle, 1998. A modifier screen in the eye reveals control genes for Kruppel activity in the Drosophila embryo. Proc. Natl. Acad. Sci. USA 9: 10779–10784.
Crick, F., 1971. General model for the chromosomes of higher organisms. Nature 234: 25–27.
Cuvier, O., C. M. Hart & U. K. Laemmli, 1998. Identification of a class of chromatin boundary elements. Mol. Cell Biol. 18: 7478–7486.
Dahmus, M. E., 1996. Reversible phosphorylation of the C-terminal domain of RNA polymerase II. J. Biol. Chem. 271: 19009–19012.
Demakov, S. A., V. F. Semeshin & I. F. Zhimulev, 1993. Cloning and molecular genetic analysis of Drosophila melanogaster interband DNA. Mol. Gen. Genet. 238: 437–443.
Frisch, M., K. Frech, A. Klingenho., K. Quandt, I. Liebich & T. Werner, 2000. A new tool for the in silico prediction of matrix attachment regions in large genomic sequences, pp. 27–34 in Proceedings of the German Conference on Bioinformatics, edited by E. Bornberg-Bauer, U. Rost, J. Stoye & M. Vingron. Logos Verlag, Berlin.
Gersh, E. S., 1975. Sites of gene activity and of inactive genes in polytene chromosomes of Diptera. J. Theor. Biol. 50: 413–428.
Gilmour, D. S. & J. T. Lis, 1986. RNA polymerase II interacts with the promoter region of the noninduced hsp-70 gene in Drosophila melanogaster cells. Mol. Cell Biol. 6: 3984–3989.
Goode, S., M. Melnick, T. B. Chou & N. Perrimon, 1996. The neurogenic genes egghead and brainiac define a novel signaling pathway essential for epithelial morphogenesis during Drosophila oogenesis. Development 122: 3863–3879.
Hancock, R., 2000. A new look at the nuclear matrix. Chromosoma 109: 219–225.
Hart, K., T. Klein & M. Wilcox, 1993. A Minute encoding a ribosomal protein enhances wing morphogenesis mutants. Mech. Dev. 43: 101–110.
Hollmann, M., 2000. Drosophila melanogaster WDS (wds)and egghead (egh)genes, complete cds. GenBank/EMBL/DDBJ 2000.2.9. AF233288.
Jamrich, M., A. L. Greenleaf & E. K. F. Bautz, 1977. Locali-zation of RNA-polymerase in Drosophila melanogaster polytene chromosomes. Proc. Nat. Acad. Sci. USA 74: 2079–2083.
Jenuwein, T. & C. D. Allis, 2001. Translating the histone code. Science 293: 1074–1080.
Jin, Y., Y. Wang, D. L. Walker, H. Dong, C. Conley, J. Johansen & K. M. Johansen, 1999. JIL-1: a novel chromosomal tandem kinase implicated in transcriptional regulation in Drosophila. Mol. Cell 4: 129—135.
Kaplan, C. D., J. R. Morris, C.-T. Wu & F. Winston, 2000. Spt5 and Spt6 are associated with active transcription and have characteristics of general elongation factors in D. melano-gaster. Genes Dev. 14: 2623–2634.
Kellum, R. & P. Schedl, 1992. A group of scs elements function as domain boundaries in enhancer-blocking assay. Mol. Cell Biol. 12: 2424–2431.
Kelley, M. R., S. Kidd, R. L. Berg & M. W. Young, 1987. Restriction of P element insertions at the Notch locus of Drosophila melanogaster. Mol. Cell Biol. 7: 1545–1548.
Keppy, D. O. & W. J. Welshons, 1977. The cytogenetics of a recessive visible mutant associated with a de ciency adja-cent to the Notch locus in Drosophila melanogaster. Genet-ics 85: 497–506.
Korge, G., I. Heide, M. Sehnert & A. Hofmann, 1980. Promoter is a important determinant of developmentally regulated pu. ng at the Sgs-4 locus of Drosophila melano-gaster. Develop. Biol. 138: 324–337.
Kozlova, T., I. F. Zhimulev & F. C. Kafatos, 1997. Molecular organization of an individual Drosophila polytene chromo-some chromomere: transcribed sequences in the 10A1-2 band. Mol. Gen. Genet. 257: 55–61.
Laemmli, U. K., E. Kas, L. Poljak & Y. Adachi, 1992. Sca. old-associated regions: cis-acting determinants of chromatin structural loops and functional domains. Curr. Opin. Genet. Dev. 2: 275–285.
Law, A., K. Hirayoshi, T. O' Brien & J. T. Lis, 1998. Direct cloning of DNA that interacts in vivo with a speci c protein: application to RNA polymerase II and sites of pausing in Drosophila. Nucl. Acids Res. 26: 919–924.
Levy, A. & M. Nöll, 1981. Chromatin fine structure of active and repressed genes. Nature 289: 198–203.
Liao, G., E. J. Rehm & G. M. Rubin, 2000. Insertion site preferences of the P transposable element in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 97: 3347–3351.
Mott, M. R. & R. J. Hill, 1986. The ultrastructural morphology of native salivary gland chromosomes of Drosophila mela-nogaster: the band-interband question. Chromosoma 94: 403–411.
O' Kane, C. J. & W. C. Gehring, 1987. Detection in situ of genomic regulatory elements in Drosophila. Proc. Natl. Acad. Sci. USA 84: 9123–9127.
Paul, J., 1972. General theory of chromosomes structure and gene activation in eukaryotes. Nature 238: 444–446.
Pirrotta, V., 1999. Transvection and chromosomal trans-interaction effects. Biochim. Biophys. Acta 1424: M1–M8.
Pirrotta, V. & Ch. Bröckl, 1984. Transcription of the Drosoph-ila white locus and some of its mutants. EMBO J. 3: 563–568.
Prokopenko, S. N. & H. J. Bellen, 2000. From phenotype to gene function: a molecular screen for novel genes required for the development of the peripheral nervous system. Dros. Res. Conf. 41: 727C.
Ramos, R. G. P., B. G. Grimwade, K. A. Wharton, T. N. Scott-gale & S. Artavanis-Tsakonas, 1989. Physical and func-tional de nition of the Drosophila Notch locus by P element transformation. Genetics 123: 337–348.
Razin, S. V., I. I. Gromova & O. V. Iarovaia, 1995. Specificity and functional signi cance of DNA interactions with the nuclear matrix: new approaches to clarify the old questions. Int. Rev. Cytol. 162B: 405–448.
Rykowski, M. C., S. J. Parmelee, D. A. Agard & J. W. Sedat, 1988. Precise determination of the molecular limits of a polytene chromosome band: regulatory sequences for the Notch gene are in the interband. Cell 54: 461–472.
Sambrook, J., E. F. Fritsch & T. Maniatis, 1989. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York.
Sass, H. & E. K. F. Bautz, 1982. Interbands of polytene chromosomes: binding sites and start points for RNA polymerase. Chromosoma 86: 77–93.
Schwartz, Yu. B., S. A. Demakov & I. F. Zhimulev, 1998. Cloning and analysis of DNA from 85D9/D10 and 86B4/ B6 interband region. Genetika 34: 905-913 (in Russian).
Schwartz, Yu. B., E. S. Ioudinkova, S. A. Demakov, S. V. Razin & I. F. Zhimulev, 1999. Interbands of Drosophila melano-gaster polytene chromosomes contain matrix association regions. J. Cell Biochem. 72: 368–372.
Schwartz, Yu. B., S. A. Demakov & I. F. Zhimulev, 2001. Polytene chromosome interband DNA is organized into nucleosomes. Mol. Genet. Genom. 265: 311–315.
Semeshin, V. F., I. F. Zhimulev & E. S. Belyaeva, 1979. Electron microscope autoradiographic study of transcriptional activ-ity of Drosophila melanogaster polytene chromosomes. Chromosoma 73: 163–177.
Semeshin, V. F., E. M. Baricheva, E. S. Belyaeva & I. F. Zhimu-lev, 1982. Electron microscopical analysis of Drosophila polytene chromosomes. I. Mapping of the 87A and 87C heat shock pu. s in development. Chromosoma 87: 229–237.
Semeshin, V. F., E. S. Belyaeva, I. F. Zhimulev, J. T. Lis, G. Richards & M. Bourouis, 1986. Electron microscopical analysis of Drosophila polytene chromosomes. IV. Mapping of morphological structures appearing as a result of transformation of DNA sequences into chromosomes. Chromosoma 93: 461–468.
Semeshin, V. F., S. A. Demakov, M. Perez Alonso, E. S. Belya-eva, J. J. Bonner & I. F. Zhimulev, 1989. Electron micro-scopical analysis of Drosophila polytene chromosomes. V. Characteristics of structures formed by transposed DNA segments of mobile elements. Chromosoma 97: 396–412.
Semeshin, V. F., V. A. Chernukhin, I. V. Shabel' nikov, L. V. Omel' yanchuk, E. S. Belyaeva & I. F. Zhimulev, 1994. Cytogenetic analysis of insertions belong to interbands of Drosophila polytene chromosomes. Genetika 30: 927-933 (in Russian).
Semeshin, V. F., R. Artero, M. Perez Alonso & V. V. Shloma, 1998. Electron microscopic in situ hybridization of digox-igenin-dUTP-labelled DNA probes with Drosophila poly-tene chromosomes. Chromosome Res. 6: 405–410.
Siden-Kiamos, I., R. D. Saunders, L. Spanos, T. Majerus, J. Treanear, C. Savakis, C. Louis, D. M. Glover, M. Ash-burner & F. C. Kafatos, 1990. Towards a physical map of the Drosophila melanogaster genome: mapping of cosmid clones within defined genomic divisions. Nucl. Acids Res. 18: 6261–6270.
Singh, G. A., J. A. Kramer & S. A. Krawetz, 1997. Mathematical model to predict regions of chromatin attachment to the nuclear matrix. Nucl. Acids Res. 25: 1419–1425 (http: // www. futuresoft. org/MAR-Wiz).
Skaer, R. J., 1977. Interband transcription in Drosophila. J. Cell Sci. 26: 251–266.
Sorsa, V., 1984. Electron microscopic mapping and ultrastruc-ture of Drosophila polytene chromosomes, 2, pp.75–107 in Insect Ultratructure, edited by King, R. C. & H. Akai. Plenum Press, New York.
Spradling, A. C., D. M. Stern, I. Kiss, J. Roote, T. Laverty & G. M. Rubin, 1995. Gene disruptions using P transposable elements: an integral component of Drosophila genome project. Proc. Natl. Acad. Sci. USA 92: 10824–10830.
Spradling, A. C., D. Stern, A. Beaton, E. J. Rehm, T. Laverty, N. Mozden, S. Misra & G. M. Rubin, 1999. The Berkeley Drosophila genome project gene disruptions project: single P element insertions mutating 25 %of vital Drosophila genes. Genetics 153: 135–177.
Udvardy, A., E. Maine & P. Schedl, 1985. The 87A chromo-mere. Identi cation of novel chromatin structures. anking the heat shock locus that may de ne the boundaries of higher order domains. J. Mol. Biol. 185: 341–358.
Vazquez, J. & P. Schedl, 1994. Sequences required for enhancer blocking activity of scs are located within two nuclease-hypersensitive regions. EMBO J. 13: 5984–5993.
Vazquez, J. & P. Schedl, 2000. Deletion of an insulator element by the mutation facet-strawberry in Drosophila melanogas-ter. Genetics 155: 1297–1311.
Vlassova, I. E., G. H. Umbetova, V. H. Zimmermann, C. Alonso, E. S. Belyaeva & I. F. Zhimulev, 1985. Immuno-fluorescence localization of DNA: RNA hybrids in Drosophila melanogaster polytene chromosomes. Chromosoma 91: 251–258.
Wang, Y., W. Zheng, Y. Jin, J. Johansen & K. M. Johansen, 2001. The JIL-1 tandem kinase mediates histone H3 phosphorylation and is required for maintenance of chro-matin structure in Drosophila. Cell 105: 433–443.
Weeks, J. R., S. E. Hardin, J. Shen, J. M. Lee & A. L. Greenleaf, 1993. Locus-specific variation in phosphorylation state of RNA polymerase II in vivo: correlations with gene activ-ity and transcript processing. Genes Dev. 7: 2329–2344.
Zhao, K., C. M. Hart & U. K. Laemmli, 1995. Visualization of chromosomal domains with Boundary Element Associated Factor BEAF-32. Cell 81: 879–889.
Zhimulev, I. F., 1996. Morphology and structure of polytene chromosomes. Adv. Genet. 34: 1–497.
Zhimulev, I. F., 1999. Genetic organization of polytene chro-mosomes. Adv. Genet. 39: 1–589.
Zhimulev, I. F. & E. S. Belyaeva, 1975. 3H-uridine labelling patterns in the Drosophila melanogaster salivary gland chromosomes X,2R and 3L. Chromosoma 49: 219–231.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Demakov, S., Gortchakov, A., Schwartz, Y. et al. Molecular and genetic organization of Drosophila melanogasterpolytene chromosomes: evidence for two types of interband regions. Genetica 122, 311–324 (2004). https://doi.org/10.1007/s10709-004-2839-0
Issue Date:
DOI: https://doi.org/10.1007/s10709-004-2839-0