Abstract
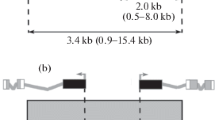
We studied whether interbands can be ectopically formed in Drosophila melanogaster polytene chromosomes. For comparative purposes, two types of P-element constructs were used. The first type was represented by P-element based insertions into compact bands. Sequences of these insertions or adjacent genomic sequences could be activated ectopically either by GAL4 or by dosage compensation machinery. In the second type, the DNA from transcriptionally silent interbands was positioned between the FRT sites, and was flanked by DNA sequences of genes that were also inactive in salivary glands. Electron microscopy analysis of salivary gland polytene chromosomes demonstrated that both types of constructs formed distinct, yet morphologically similar interbands. Notably, the second class of transposon insertions appeared in polytene chromosomes as two bands separated by one interband. Excision of interband material from such insertions resulted in fusion of newly appeared bands into a single band. We were able to confirm by molecular means that the DNA sequences in integrated constructs were intact, that chromatin organization of this DNA mimicked that of native interbands, and that it was accurately excised from the constructs by FLP. Thus, we demonstrate that transfer of interband DNA into a silent genetic environment does not compromise interband formation. Our results do not support the idea of the existence of distinct cytogenetic “band + interband” units, furthermore, they suggest the autonomy of the decompacted state of interbands.







Similar content being viewed by others
References
Beermann W (1972) Chromomeres and genes. In: Beermann W (ed) Results and problems in cell differentiation, vol. 4. Springer, Berlin, Heidelberg, New York, pp 1–33
Brennecke J, Hipfner DR Stark A, Russell RB, Cohen SM (2003) bantam encodes a developmentally regulated microRNA that controls cell proliferation and regulates the proapoptotic gene hid in Drosophila. Cell 113:25–36
Cherbas L, Hu X, Zhimulev I, Belyaeva E, Cherbas P (2003) EcR isoforms in Drosophila: testing tissue-specific requirements by targeted blockade and rescue. Development 130(2):271–284
Clark RF, Wagner CR, Craig CA, Elgin RSC (1991) Distribution of chromosomal proteins in polytene chromosomes of Drosophila. Methods Cell Biol 35:203–227
Crick F (1971) General model for chromosomes of higher organisms. Nature 234:25–27
Demakov S, Gortchakov A, Schwartz Y, Semeshin V, Campuzano S, Modolell J, Zhimulev I (2004) Molecular and genetic organization of Drosophila melanogaster polytene chromosomes: evidence for two types of interband regions. Genetica 122:311–324
Deng H, Zhang W, Bao X, Martin JN, Girton J, Johansen J, Johansen KM (2005) The JIL-1 kinase regulates the structure of Drosophila polytene chromosomes. Chromosoma 114(3):173–182
Eggert H, Gortchakov A, Saumweber H (2004) Identification of the Drosophila interband-specific protein Z4 as a DNA-binding zinc-finger protein determining chromosomal structure. J Cell Sci 117:4253–4264
Gall JG, Pardue ML (1969) Formation and detection of RNA–DNA hybrid molecules in cytological preparations. Proc Natl Acad Sci USA 63:378–83
Georgiev PG, Muravyova EE, Golovnin AK, Gracheva EM, Belenkaya TYu (2000) Insulators and long-distance interaction between regulatory elements in higher eukaryotes. Genetika 36:1588–1597 (in Russian)
Gilbert MK, Tan YY, Hart CM (2006) The Drosophila boundary element-associated factors BEAF-32A and BEAF-32B affect chromatin structure. Genetics 173:1365–1375
Golic MM, Rong YS, Peteresen RB, Lindquist SL, Golic KG (1997) FLP-mediated DNA mobilization to specific target sites in Drosophila chromosomes. Nucleic Acids Res 25:3665–3671
Gortchakov AA, Demakov SA, Schwartz YuB (2003) Construction of pFRT, a convenient Drosophila transformation vector with the functional FRT sites. Mol Biol 37(5):695–698 (in Russian)
Gortchakov AA, Eggert H, Gan M, Mattow J, Zhimulev IF, Saumweber H (2005) Chriz, a chromodomain protein specific for the interbands of Drosophila melanogaster polytene chromosomes. Chromosoma 114:54–66
Hart CM, Zhao K, Laemmli UK (1997) The scs’ boundary element: characterization of boundary element-associated factors. Mol Cell Biol 17(2):999–1009
Jamrich M, Haars R, Wulf E, Bautz FA (1977) Correlation of RNA polymerase B and transcriptional activity in the chromosomes of Drosophila melanogaster. Chromosoma 64:319–326
Jin Y, Wang Y, Walker DL, Dong H, Conley C, Johansen J, Johansen KM (1999) JIL-1: a novel chromosomal tandem kinase implicated in transcriptional regulation in Drosophila. Mol Cell 4:129–135
Kaplan CD, Morris JR, Wu C, Winston F (2000) Spt5 and Apt6 are associated with active transcription and have characteristics of general elongation factors in D. melanogaster. Genes Dev 14(20):2623–2634
Kelley RL, Meller VH, Gordadze PR, Roman G, Davis RL, Kuroda MI (1999) Epigenetic spreading of the Drosophila dosage compensation complex from roX RNA genes into flanking chromatin. Cell 98:513–522
Kelley RL, Kuroda MI (2003) The Drosophila roX1 RNA gene can overcome silent chromatin by recruiting the male-specific lethal dosage compensation complex. Genetics 164:565–574
Kozlova T, Zhimulev IF, Kafatos FC (1997) Molecular organization of an individual Drosophila polytene chromosome chromomere: transcribed sequences in the 10A1-2 band. Mol Gen Genet 257:55–61
Lindsley DL, Zimm GG (1992) The genome of Drosophila melanogaster. Academic Press, San Diego, California
Lis JT, Mason P, Peng J, Price DH, Werner J (2000) P-TEFb kinase recruitment and function at heat shock loci. Genes Dev 14:792–803
O’Kane C, Gehring WJ (1987) Detection in situ of genomic regulatory elements in Drosophila. Proc Natl Acad Sci USA 84:9123–9127
Paul J (1972) General theory of chromosomes structure and gene activation in eukaryotes. Nature 238:444–446
Raisin S, Pantalacci S, Breittmayer JP, Leopold P (2003) A new genetic locus controlling growth and proliferation in Drosophila melanogaster. Genetics 164:1015–1025
Ramos RGP, Grimwade BG, Wharton KA, Scottgale TN, Artavanis-Tsakonas S (1989) Physical and functional definition of the Drosophila Notch locus by P element transformation. Genetics 123:337–348
Rath U, Ding Y, Deng H, Qi H, Bao X, Zhang W, Girton J, Johansen J, Johansen KM. (2006) The chromodomain protein, Chromator, interacts with JIL-1 kinase and regulates the structure of Drosophila polytene chromosomes. J Cell Sci 119:2332–2341
Rorth P (1996) A modular misexpression screen in Drosophila detecting tissue-specific phenotypes. Proc Natl Acad Sci USA 93:12418–12422
Rubin GM, Spradling AC (1982) Genetic transformation of Drosophila with transposable element vectors. Science 218:348–353
Rykowski MC, Parmelee SJ, Agard DA, Sedat JW (1988) Precise determination of molecular limits of polytene chromosome band: regulatory sequences for the Notch gene are in the interband. Cell 54:461–472
Sambrook J, Fritsch EF, Maniatis T (2004) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York
Sass H, Bautz EKF (1982) Immunoelectron microscopic localization of RNA polymerase B on isolated polytene chromosomes of Chironomus tetans. Chromosoma 85:633–642
Schwartz YuB, Demakov SA, Zhimulev IF (1998) Cloning and analysis of DNA from 85D9/D10 and 86B4/B6 interband region. Genetika 34:905–913 (in Russian)
Schwartz YuB, Ioudinkova ES, Demakov SA, Razin SV, Zhimulev IF (1999) Interbands of Drosophila melanogaster polytene chromosomes contain matrix association regions. J Cell Biochem 72:368–372
Schwartz YuB, Demakov SA, Zhimulev IF (2001) Polytene chromosome interband DNA is organized into nucleosomes. Mol Genet Genom 265:311–315
Shaffer CD, Wuller JM, Elgin SCR (1994) Preparation of Drosophila nuclei. Methods Cell Biol 44:185–189
Semeshin VF, Zhimulev IF, Belyaeva ES (1979) Electron microscope autoradiographic study of transcriptional activity of Drosophila melanogaster polytene chromosomes. Chromosoma 73:163–177
Semeshin VF, Belyaeva ES, Zhimulev IF, Lis JT, Richards G, Bourouis M (1986) Electron microscopical analysis of Drosophila polytene chromosomes. IV. Mapping of morphological structures appearing as a result of transformation of DNA sequences into chromosomes. Chromosoma 93:461–468
Semeshin VF, Demakov SA, Perez Alonso M, Belyaeva ES, Bonner JJ, Zhimulev IF (1989) Electron microscopical analysis of Drosophila polytene chromosomes. V. Characteristics of structures formed by transposed DNA segments of mobile elements. Chromosoma 97:396–412
Semeshin VF, Belyaeva ES, Zhimulev IF (2001) Electron microscope mapping of pericentric and intercalary heterochromatic regions of the polytene chromosomes of the mutant Supressor of underreplication in Drosophila melanogaster. Chromosoma 110:487–500
Semeshin VF, Belyaeva ES, Shloma VV, Zhimulev IF (2004) Electron microscopy of polytene chromosomes. Methods Mol Biol 247:305–324
Sorsa V (1984) Electron microscopic mapping and ultrastructure of Drosophila polytene chromosomes. In: King RC, Akai H (eds) Insect ultrastructure, vol. 2. Plenum Press, New York, pp 75–107
Udvardy A (1999) Dividing the empire: boundary chromatin elements delimit the territory of enhancers. EMBO J 18(1):1–8
Vazquez J, Schedl P (2000) Deletion of an insulator element by the mutation facet-strawberry in Drosophila melanogaster. Genetics 155:1297–1311
Vlassova IE, Umbetova GH, Zimmermann VH, Alonso C, Belyaeva ES, Zhimulev IF (1985) Immunofluorescence localization of DNA:RNA hybrids in Drosophila melanogaster polytene chromosomes. Chromosoma 91:251–258
Wang Y, Zheng W, Jin Y, Johansen J, Johansen KM (2001) The JIL-1 tandem kinase mediates histone H3 phosphorylation and is required for maintenance of chromatin structure in Drosophila. Cell 105:433–443
Zhao K, Hart CM, Laemmli UK (1995) Visualization of chromosomal domains with Boundary Element Associated Factor BEAF-32. Cell 81:879–889
Zhimulev IF (1996) Morphology and structure of polytene chromosomes. Adv Genet 34:1–490
Zhimulev IF (1999) Genetic organization of polytene chromosomes. Adv Genet 39:1–589
Zhimulev IF, Belyaeva ES (1975) Proposals to the problem of structural and functional organization of polytene chromosomes. Theor Appl Genet 45:335–340
Zhimulev IF, Belyaeva ES, Semeshin VF, Koryakov DE, Demakov SA, Demakova 0V, Pokholkova GV, Andreyeva EN (2004) Polytene chromosomes: 70 years of genetic research. Int Rev Cytol 241:203–275
Zimin PI, Gortchakov AA, Demakov SA, Zhimulev IF (2004) A new construct for cloning DNA and modeling the structure of Drosophila melanogaster polytene chromosomes. Mol Biol 38(2):250–255 (in Russian)
Acknowledgements
The authors are grateful to Mitzi Kuroda, Lucy Cherbas and Ekaterina Savitskaya for providing us with fly stocks. This work was supported by the Russian Foundation for Basic Research, grant NN 06-04-48387 and 06-04-49305-a; the program Leading Scientific Schools, grant no. 942.2006.4, and Molecular and Cellular Biology program no 10.1 from RAS.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Semeshin, V.F., Demakov, S.A., Shloma, V.V. et al. Interbands behave as decompacted autonomous units in Drosophila melanogaster polytene chromosomes. Genetica 132, 267–279 (2008). https://doi.org/10.1007/s10709-007-9170-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10709-007-9170-5