Abstract
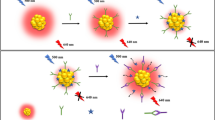
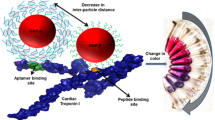
Human cardiac troponin I (hcTnI) and troponin T (hcTnT) are the biomarkers of choice for the diagnosis of cardiac diseases. In an effort to improve assay sensitivity, in this study we developed a novel approach to simultaneously detect hcTnI and hcTnT in homogenous solutions by monitoring enhanced-fluorescence-anisotropy changes. Specifically, our design was based on a competition assay by measuring anisotropy change of fluorophore-labeled peptides bound to primary monoclonal antibodies in the presence of nano-gold-modified secondary antibody in response to the presence of target proteins. Enhanced-fluorescence-anisotropy resulted from interaction between the primary antibody and the nano-gold-labeled secondary antibody, which significantly increased the size and decreased tumbling motion of the complex of peptide-antibodies. The measurements were performed to detect hcTnI and hcTnT either individually or simultaneously in a homogenous buffer solution and in the solutions containing human plasma. Our results showed that when fluorescence emission was monitored at a single wavelength selected by a monochromator the assay at all experimental conditions had excellent linear response to the target proteins within the concentration range of 0.5–40 nM. The detection limit is 0.5 nM for both hcTnI and hcTnT in the presence of human plasma. However, when fluorescence emission was monitored using a cutoff filter, the linear response of the assay to the target proteins is within 15–500 pM. The detection limit is 15 pM which is close to the recommended 99th percentile cutoff point for concentrations of hcTnI and hcTnT tests to discriminate healthy and diseased conditions. Homogenous nature, rapid response time, and easy implementation of our assay design make it a useful tool for disease biomarker and protein sensing.






Similar content being viewed by others
Abbreviations
- hcTnI:
-
human cardiac troponin I
- hcTnT:
-
human cardiac troponin T
- AMI:
-
acute myocardial infarction
References
Alpert JS, Thygesen K, Antman E, Bassand JP (2000) Myocardial infarction redefined—a consensus document of The Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. J Am Coll Cardiol 36:959–969
Babuin L, Jaffe AS (2005) Troponin: the biomarker of choice for the detection of cardiac injury. CMAJ 173:1191–1202
Katus HA, Remppis A, Looser S, Hallermeier K, Scheffold T, Kubler W (1989) Enzyme linked immuno assay of cardiac troponin T for the detection of acute myocardial infarction in patients. J Mol Cell Cardiol 21:1349–1353
Vo-Dinh T, Cullum B (2000) Biosensors and biochips: advances in biological and medical diagnostics. Fresenius J Anal Chem 366:540–551
Wu AH, Feng YJ, Moore R, Apple FS, McPherson PH, Buechler KF, Bodor G (1998) Characterization of cardiac troponin subunit release into serum after acute myocardial infarction and comparison of assays for troponin T and I. American Association for Clinical Chemistry Subcommittee on cTnI Standardization. Clin Chem 44:1198–1208
Mohammed AA, Januzzi JL Jr (2010) Clinical applications of highly sensitive troponin assays. Cardiol Rev 18:12–19
Dutra RF, Mendes RK, Lins da Silva V, Kubota LT (2007) Surface plasmon resonance immunosensor for human cardiac troponin T based on self-assembled monolayer. J Pharm Biomed Anal 43:1744–1750
Wei J, Mu Y, Song D, Fang X, Liu X, Bu L, Zhang H, Zhang G, Ding J, Wang W, Jin Q, Luo G (2003) A novel sandwich immunosensing method for measuring cardiac troponin I in sera. Anal Biochem 321:209–216
O’Regan TM, Pravda M, O’Sullivan CK, Guilbault GG (2002) Development of a disposable immunosensor for the detection of human heart fatty-acid binding protein in human whole blood using screen-printed carbon electrodes. Talanta 57:501–510
Grant SA, Pierce ME, Lichlyter DJ, Grant DA (2005) Effects of immobilization on a FRET immunosensor for the detection of myocardial infarction. Anal Bioanal Chem 381:1012–1018
Hong B, Kai J, Ren Y, Han J, Zou Z, Ahn CH, Kang KA (2008) Highly sensitive rapid, reliable, and automatic cardiovascular disease diagnosis with nanoparticle fluorescence enhancer and mems. Adv Exp Med Biol 614:265–273
Wang J, Hong B, Kai J, Han J, Zou Z, Ahn CH, Kang KA (2009) Mini sensing chip for point-of-care acute myocardial infarction diagnosis utilizing micro-electro-mechanical system and nano-technology. Adv Exp Med Biol 645:101–107
Vestergaard MD, Kerman K, Tamiya E (2007) An overview of label-free electrochemical protein sensors. Sensors 7:3442–3458
Gryczynski Z, Abugo OO, Lakowicz JR (1999) Polarization sensing of fluorophores in tissues for drug compliance monitoring. Anal Biochem 273:204–211
Lakowicz JR, Gryczynski I, Gryczynski Z (2000) Novel fluorescence sensing methods for high throughput screening. J Biomol Screen 5:123–132
Mann TL, Krull UJ (2003) Fluorescence polarization spectroscopy in protein analysis. Analyst 128:313–317
Dong WJ, Xing J, Villain M, Hellinger M, Robinson JM, Chandra M, Solaro RJ, Umeda PK, Cheung HC (1999) Conformation of the regulatory domain of cardiac muscle troponin C in its complex with cardiac troponin I. J Biol Chem 274:31382–31390
Lakowicz JR (1999) Principles of fluorescence spectroscopy. Kluwer, New York
Kociol RD, Pang PS, Gheorghiade M, Fonarow GC, O’Connor CM, Felker GM (2010) Troponin elevation in heart failure prevalence, mechanisms, and clinical implications. J Am Coll Cardiol 56:1071–1078
Katagiri T, Kobayashi Y, Sasai Y, Toba K, Niitani H (1981) Alterations in cardiac troponin subunits in myocardial infarction. Jpn Heart J 22:653–664
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Qiao, Y., Tang, H., Munske, G.R. et al. Enhanced Fluorescence Anisotropy Assay for Human Cardiac Troponin I and T Detection. J Fluoresc 21, 2101–2110 (2011). https://doi.org/10.1007/s10895-011-0909-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-011-0909-0