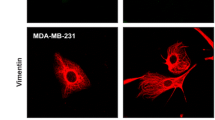
Abstract
One fundamental difference between normal and transformed cells is the way they interact with their immediate environment. Exploring this difference is crucial for understanding the pathobiology of cancer progression. Benign epithelial tumors are constrained by a surrounding stroma consisting, among other cells, of fibroblasts embedded within fibrillar three-dimensional matrices. However, at a critical point in tumor progression, tumor cells become altered and overcome the barrier, inducing changes in the stroma, which promote, rather than impede, tumor progression. Inherited or acquired genetic aberrations affecting mammary gland epithelia are usually blamed for promoting neoplasia in individuals at “high risk” for breast cancer. However, in addition to these epithelial aberrations certain individuals possess permissive breast stroma. The occurrence of this permissive stroma results in a predisposition for cancer initiation or progression. Here we review stromagenic stages, experimental 3D systems, and discuss digital imaging analyses suitable for uncovering the mechanisms behind fibroblastic breast stromagenesis.
Similar content being viewed by others
References
Noel A, Kebers F, Maquoi E, Foidart JM. Cell–cell and cell–matrix interactions during breast cancer progression. Curr Top Pathol 1999;93:183–193.
Tuxhorn JA, Ayala GE, Rowley DR. Reactive stroma in prostate cancer progression. J Urol 2001;166(6):2472–83.
Kunz-Schughart LA, Knuechel R. Tumor-associated fibroblasts (Part II): Functional impact on tumor tissue. Histol Histopathol 2002;17(2):623–37.
Kunz-Schughart LA, Knuechel R. Tumor-associated fibroblasts (Part I): Active stromal participants in tumor development and progression? Histol Histopathol 2002;17(2):599–621.
Schaefer D, Jurgensmeier JM, Bauer G. Catechol interferes with TGF-beta-induced elimination of transformed cells by normal cells: Implications for the survival of transformed cells during carcinogenesis. Int J Cancer 1995;60(4):520–6.
Bauer G. Elimination of transformed cells by normal cells: A novel concept for the control of carcinogenesis. Histol Histopathol 1996;11(1):237–55.
Park CC, Bissell MJ, Barcellos-Hoff MH. The influence of the microenvironment on the malignant phenotype. Mol Med Today 2000;6(8):324–9.
Elenbaas B, Weinberg RA. Heterotypic signaling between epithelial tumor cells and fibroblasts in carcinoma formation. Exp Cell Res 2001;264(1):169–84.
Sung SY, Chung LW. Prostate tumor–stroma interaction: Molecular mechanisms and opportunities for therapeutic targeting. Differentiation 2002;70(9–10):506–21.
Brown RA, Sethi KK, Gwanmesia I, Raemdonck D, Eastwood M, Mudera V. Enhanced fibroblast contraction of 3D collagen lattices and integrin expression by TGF-beta1 and -beta3: Mechanoregulatory growth factors? Exp Cell Res 2002;274(2):310–22.
Geiger B, Bershadsky A, Pankov R, Yamada KM. Transmembrane crosstalk between the extracellular matrix and the cytoskeleton. Nat Rev Mol Cell Biol 2001;2(11):793–805.
Graf R, Freyberg M, Kaiser D, Friedl P. Mechanosensitive induction of apoptosis in fibroblasts is regulated by thrombospondin-1 and integrin associated protein (CD47). Apoptosis 2002;7(6):493–8.
Jalali S, del Pozo MA, Chen K-D, Miao H, Li Y-S, Schwartz MA, et al. Integrin-mediated mechanotransduction requires its dynamic interaction with specific extracellular matrix (ECM) ligands. PNAS 2001;98(3):1042–46.
Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol 2002;3(5):349–63.
Zhang HJ, Cai SX, Lu X. Role of integrins in cellular responses to mechanical stress. Prog Biochem Biophys 2002;29(3):359–62.
Katsumi A, Orr AW, Tzima E, Schwartz MA. Integrins in mechanotransduction. J Biol Chem 2004;279(13):12001–4.
Cukierman E, Pankov R, Stevens DR, Yamada KM. Taking cell-matrix adhesions to the third dimension. Science 2001;294(5547):1708–12.
Ingber DE. Cancer as a disease of epithelial–mesenchymal interactions and extracellular matrix regulation. Differentiation 2002;70(9–10):547–60.
Schatzmann F, Marlow R, Streuli CH. Integrin signaling and mammary cell function. J Mammary Gland Biol Neoplasia 2003;8(4):395–408.
Cunha GR, Matrisian LM. It’s not my fault, blame it on my microenvironment. Differentiation 2002;70(9–10):469–72.
Abelev GI. Differentiation antigens: Dependence on carcinogenesis mechanisms and tumor progression (a hypothesis). Mol Biol 2003;37(1):2–8.
Jones JL, Shaw JA, Pringle JH, Walker RA. Primary breast myoepithelial cells exert an invasion-suppressor effect on breast cancer cells via paracrine down-regulation of MMP expression in fibroblasts and tumour cells. J Pathol 2003;201(4):562–72.
Rose-Hellekant TA, Gilchrist K, Sandgren EP. Strain background alters mammary gland lesion phenotype in transforming growth factor-{alpha} transgenic mice. Am J Pathol 2002;161(4):1439–47.
Saez E, Rosenfeld J, Livolsi A, Olson P, Lombardo E, Nelson M, et al. PPAR gamma signaling exacerbates mammary gland tumor development. Genes Dev 2004;18(5):528–40.
Kuperwasser C, Chavarria T, Wu M, Magrane G, Gray JW, Carey L, et al. Reconstruction of functionally normal and malignant human breast tissues in mice. PNAS 2004;101(14):4966–71.
Laconi E, Oren R, Mukhopadhyay DK, Hurston E, Laconi S, Pani P, et al. Long-term, near-total liver replacement by transplantation of isolated hepatocytes in rats treated with retrorsine. Am J Pathol 1998;153(1):319–29.
Maffini MV, Soto AM, Calabro JM, Ucci AA, Sonnenschein C. The stroma as a crucial target in rat mammary gland carcinogenesis. J Cell Sci 2004;117(8):1495–1502.
Weaver VM, Gilbert P. Watch thy neighbor: Cancer is a communal affair. J Cell Sci 2004;117(8):1287–90.
Shekhar MP, Werdell J, Santner SJ, Pauley RJ, Tait L. Breast stroma plays a dominant regulatory role in breast epithelial growth and differentiation: Implications for tumor development and progression. Cancer Res 2001;61(4):1320–26.
Moinfar F, Man YG, Arnould L, Bratthauer GL, Ratschek M, Tavassoli FA. Concurrent and independent genetic alterations in the stromal and epithelial cells of mammary carcinoma: Implications for tumorigenesis. Cancer Res 2000;60(9):2562–66.
Bissell MJ, Radisky D. Putting tumours in context. Nat Rev Cancer 2001;1(1):46–54.
Bissell MJ, Radisky DC, Rizki A, Weaver VM, Petersen OW. The organizing principle: Microenvironmental influences in the normal and malignant breast. Differentiation 2002;70(9–10):537–46.
Mueller MM, Fusenig NE. Tumor–stroma interactions directing phenotype and progression of epithelial skin tumor cells. Differentiation 2002;70(9–10):486–97.
Wong YC, Tam NN. Dedifferentiation of stromal smooth muscle as a factor in prostate carcinogenesis. Differentiation 2002;70(9–10):633–45.
Cheng JD, Weiner LM. Tumors and their microenvironments: Tilling the soil. Commentary re: A. M. Scott et al., A phase I dose-escalation study of sibrotuzumab in patients with advanced or metastatic fibroblast activation protein-positive cancer. Clin Cancer Res 2003;9:1639–47, 9(5):1590–95.
Boyd RS, Balkwill FR. MMP-2 release and activation in ovarian carcinoma: The role of fibroblasts. Br J Cancer 1999;80(3–4):315–21.
Chrenek MA, Wong P, Weaver VM. Tumour-stromal interactions: Integrins and cell adhesions as modulators of mammary cell survival and transformation. Breast Cancer Res 2001;3(4):224–9.
Tang Y, Kesavan P, Nakada MT, Yan L. Tumor–stroma interaction: Positive feedback regulation of extracellular matrix metalloproteinase inducer (EMMPRIN) expression and matrix metalloproteinase-dependent generation of soluble EMMPRIN. Mol Cancer Res 2004;2(2):73–80.
Bisson C, Blacher S, Polette M, Blanc JF, Kebers F, Desreux J, et al. Restricted expression of membrane type 1-matrix metalloproteinase by myofibroblasts adjacent to human breast cancer cells. Int J Cancer 2003;105(1):7–13.
Behrens P, Rothe M, Wellmann A, Krischler J, Wernert N. The Ets-1 transcription factor is up-regulated together with MMP 1 and MMP 9 in the stroma of pre-invasive breast cancer. J Pathol 2001;194(1):43–50.
Werb Z, Sympson CJ, Alexander CM, Thomasset N, Lund LR, MacAuley A, et al. Extracellular matrix remodeling and the regulation of epithelial–stromal interactions during differentiation and involution. Kidney Int 1996;54(Suppl.):S68–74.
Singer CF, Kronsteiner N, Marton E, Kubista M, Cullen KJ, Hirtenlehner K, et al. MMP-2 and MMP-9 expression in breast cancer-derived human fibroblasts is differentially regulated by stromal–epithelial interactions. Breast Cancer Res Treat 2002;72(1):69–77.
Mari BP, Anderson IC, Mari SE, Ning Y, Lutz Y, Kobzik L, et al. Stromelysin-3 is induced in tumor/stroma cocultures and inactivated via a tumor-specific and basic fibroblast growth factor-dependent mechanism. J Biol Chem 1998;273(1):618–26.
Saad S, Gottlieb DJ, Bradstock KF, Overall CM, Bendall LJ. Cancer cell-associated fibronectin induces release of matrix metalloproteinase-2 from normal fibroblasts. Cancer Res 2002;62(1):283–9.
Heppner KJ, Matrisian LM, Jensen RA, Rodgers WH. Expression of most matrix metalloproteinase family members in breast cancer represents a tumor-induced host response. Am J Pathol 1996;149(1):273–82.
Maxhimer JB, Quiros RM, Stewart R, Dowlatshahi K, Gattuso P, Fan M, et al. Heparanase-1 expression is associated with the metastatic potential of breast cancer. Surgery 2002;132(2):326–33.
Koblinski JE, Dosescu J, Sameni M, Moin K, Clark K, Sloane BF. Interaction of human breast fibroblasts with collagen I increases secretion of procathepsin B. J Biol Chem 2002;277(35):32220–7.
Martens JW, Sieuwerts AM, Vries JB, Bosma PT, Swiggers SJ, Klijn JG, et al. Aging of stromal-derived human breast fibroblasts might contribute to breast cancer progression. Thromb Haemost 2003;89(2):393–404.
Haslam S, Woodward T. Reciprocal regulation of extracellular matrix proteins and ovarian steroid activity in the mammary gland. Breast Cancer Res 2001;3(6):365–72.
Wernert N, Locherbach C, Wellmann A, Behrens P, Hugel A. Presence of genetic alterations in microdissected stroma of human colon and breast cancers. J Mol Med 2000;78(7):B30.
Sangaletti S, Stoppacciaro A, Guiducci C, Torrisi MR, Colombo MP. Leukocyte, rather than tumor-produced SPARC, determines stroma and collagen Type IV deposition in mammary carcinoma. J Exp Med 2003;198(10):1475–85.
Iyengar P, Combs TP, Shah SJ, Gouon-Evans V, Pollard JW, Albanese C, et al. Adipocyte-secreted factors synergistically promote mammary tumorigenesis through induction of anti-apoptotic transcriptional programs and proto-oncogene stabilization. Oncogene 2003;22(41):6408–23.
Walpita D, Hay E. Studying actin-dependent processes in tissue culture. Nat Rev Mol Cell Biol 2002;3(2):137–41.
Thiery JP. Cell adhesion in cancer. Comptes Rendus Physique 2003;4(2):289–304.
Zamir E, Katz M, Posen Y, Erez N, Yamada KM, Katz BZ, et al. Dynamics and segregation of cell-matrix adhesions in cultured fibroblasts. Nat Cell Biol 2000;2(4):191–6.
Pankov R, Cukierman E, Katz BZ, Matsumoto K, Lin DC, Lin S, et al. Integrin dynamics and matrix assembly. Tensin-dependent translocation of alpha(5)beta(1) integrins promotes early fibronectin fibrillogenesis. J Cell Biol 2000;148(5):1075–90.
Cukierman E, Pankov R, Yamada KM. Cell interactions with three-dimensional matrices. Curr Opin Cell Biol 2002;14(5):633–40.
Lee JW, Juliano R. Mitogenic signal transduction by integrin- and growth factor receptor-mediated pathways. Mol Cells 2004;17(2):188–202.
Hasebe T, Imoto S, Ogura T, Mukai K. Significance of basic fibroblast growth factor and fibroblast growth factor receptor protein expression in the formation of fibrotic focus in invasive ductal carcinoma of the breast. Jpn J Cancer Res 1997;88(9):877–85.
Hasebe T, Sasaki S, Imoto S, Ochiai A. Highly proliferative fibroblasts forming fibrotic focus govern metastasis of invasive ductal carcinoma of the breast. Mod Pathol 2001;14(4):325–37.
Alowami S, Troup S, Al-Haddad S, Kirkpatrick I, Watson P. Mammographic density is related to stroma and stromal proteoglycan expression. Breast Cancer Res 2003;5(5):R129–135.
Olumi AF, Grossfeld GD, Hayward SW, Carroll PR, Tlsty TD, Cunha GR. Carcinoma-associated fibroblasts direct tumor progression of initiated human prostatic epithelium. Cancer Res 1999;59(19):5002–11.
Pupa SM, Menard S, Forti S, Tagliabue E. New insights into the role of extracellular matrix during tumor onset and progression. J Cell Physiol 2002;192(3):259–67.
Skobe M, Fusenig NE. Tumorigenic conversion of immortal human keratinocytes through stromal cell activation. Proc Natl Acad Sci USA 1998;95(3):1050–55.
Hayashi N, Cunha GR, Wong YC. Influence of male genital tract mesenchymes on differentiation of Dunning prostatic adenocarcinoma. Cancer Res 1990;50(15):4747–54.
Wong YC, Cunha GR, Hayashi N. Effects of mesenchyme of the embryonic urogenital sinus and neonatal seminal vesicle on the cytodifferentiation of the Dunning tumor: Ultrastructural study. Acta Anat (Basel) 1992;143(2):139–50.
Garin-Chesa P, Old LJ, Rettig WJ. Cell surface glycoprotein of reactive stromal fibroblasts as a potential antibody target in human epithelial cancers. Proc Natl Acad Sci USA 1990;87(18):7235–69.
Huber MA, Kraut N, Park JE, Schubert RD, Rettig WJ, Peter RU, et al. Fibroblast activation protein: Differential expression and serine protease activity in reactive stromal fibroblasts of melanocytic skin tumors. J Invest Dermatol 2003;120(2):182–8.
Rettig WJ, Chesa PG, Beresford HR, Feickert HJ, Jennings MT, Cohen J, et al. Differential expression of cell surface antigens and glial fibrillary acidic protein in human astrocytoma subsets. Cancer Res 1986;46(12 Pt 1):6406–12.
Rettig WJ, Garin-Chesa P, Healey JH, Su SL, Ozer HL, Schwab M, et al. Regulation and heteromeric structure of the fibroblast activation protein in normal and transformed cells of mesenchymal and neuroectodermal origin. Cancer Res 1993;53(14):3327–35.
Cheng JD, Dunbrack RL, Jr., Valianou M, Rogatko A, Alpaugh RK, Weiner LM. Promotion of tumor growth by murine fibroblast activation protein, a serine protease, in an animal model. Cancer Res. 2002;62(16):4767–72.
Mersmann M, Schmidt A, Rippmann JF, Wuest T, Brocks B, Rettig WJ, et al. Human antibody derivatives against the fibroblast activation protein for tumor stroma targeting of carcinomas. Int J Cancer 2001;92(2):240–8.
Corsini C, Mancuso P, Paul S, Burlini A, Martinelli G, Pruneri G, et al. Stroma cells: A novel target of Herceptin activity. Clin Cancer Res 2003;9(5):1820–25.
Noel A, Foidart JM. The role of stroma in breast carcinoma growth in vivo. J Mammary Gland Biol Neoplasia 1998;3(2):215–25.
Kunz-Schughart LA, Heyder P, Schroeder J, Knuechel R. A heterologous 3-d coculture model of breast tumor cells and fibroblasts to study tumor-associated fibroblast differentiation. Exp Cell Res 2001;266(1):74–86.
Cunha GR, Hayward SW, Wang YZ. Role of stroma in carcinogenesis of the prostate. Differentiation 2002;70(9–10):473–85.
Fidler IJ. The organ microenvironment and cancer metastasis. Differentiation 2002;70(9–10):498–505.
Arihiro K, Oda H, Kaneko M, Inai K. Cytokines facilitate chemotactic motility of breast carcinoma cells. Breast Cancer 2000;7(3):221–30.
Valenti MT, Sartore S, Azzarello G, Balducci E, Amadio M, Sandri M, et al. Human fibroblasts from normal and malignant breast tissue grown in vitro show a distinct senescence profile and telomerase activity. Histochem J 2002;34(8–9):403–10.
Schor AM, Rushton G, Ferguson JE, Howell A, Redford J, Schor SL. Phenotypic heterogeneity in breast fibroblasts: Functional anomaly in fibroblasts from histologically normal tissue adjacent to carcinoma. Int J Cancer 1994;59(1):25–32.
Ronnov-Jessen L, Petersen OW, Koteliansky VE, Bissell MJ. The origin of the myofibroblasts in breast cancer. Recapitulation of tumor environment in culture unravels diversity and implicates converted fibroblasts and recruited smooth muscle cells. J Clin Invest 1995;95(2):859–73.
Thomas GJ, Hart IR, Speight PM, Marshall JF. Binding of TGF-beta1 latency-associated peptide (LAP) to alpha(v)beta6 integrin modulates behaviour of squamous carcinoma cells. Br J Cancer 2002;87(8):859–67.
Hauptmann S, Siegert A, Berger S, Denkert C, Kobel M, Ott S, et al. Regulation of cell growth and the expression of extracellular matrix proteins in colorectal adenocarcinoma: A fibroblast-tumor cell coculture model to study tumor–host interactions in vitro. Eur J Cell Biol 2003;82(1):1–8.
Kaarteenaho-Wiik R, Soini Y, Pollanen R, Paakko P, Kinnula VL. Over-expression of tenascin-C in malignant pleural mesothelioma. Histopathology 2003;42(3):280–91.
Slater MD, Lauer C, Gidley-Baird A, Barden JA. Markers for the development of early prostate cancer. J Pathol 2003;199(3):368–77.
De Santis R, Anastasi AM, D’Alessio V, Pelliccia A, Albertoni C, Rosi A, et al. Novel antitenascin antibody with increased tumour localisation for Pretargeted Antibody-Guided RadioImmunoTherapy (PAGRIT(R)). Br J Cancer 2003;88(7):996–1003.
Kalembeyi I, Inada H, Nishiura R, Imanaka-Yoshida K, Sakakura T, Yoshida T. Tenascin-C upregulates matrix metalloproteinase-9 in breast cancer cells: Direct and synergistic effects with transforming growth factor beta1. Int J Cancer 2003;105(1):53–60.
Dvorak HF. Tumors: Wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med 1986;315(26):1650–59.
Seemayer TA, Lagace R, Schurch W, Thelmo WL. The myofibroblast: Biologic, pathologic, and theoretical considerations. Pathol Annu 1980;15(Pt. 1):443–70.
Barsky SH, Green WR, Grotendorst GR, Liotta LA. Desmoplastic breast carcinoma as a source of human myofibroblasts. Am J Pathol 1984;115(3):329–33.
Ariga N, Sato E, Ohuchi N, Nagura H, Ohtani H. Stromal expression of fibroblast activation protein/seprase, a cell membrane serine proteinase and gelatinase, is associated with longer survival in patients with invasive ductal carcinoma of breast. Int J Cancer 2001;95(1):67–72.
Wiksten JP, Lundin J, Nordling S, Lundin M, Kokkola A, von Boguslawski K, et al. Tenascin-C expression correlates with prognosis in gastric cancer. Oncology 2003;64(3):245–50.
Powell DW, Mifflin RC, Valentich JD, Crowe SE, Saada JI, West AB. Myofibroblasts. I. Paracrine cells important in health and disease. Am J Physiol 1999;277(1 Pt. 1):C1–9.
Matsuura H, Hakomori S. The oncofetal domain of fibronectin defined by monoclonal antibody FDC-6: Its presence in fibronectins from fetal and tumor tissues and its absence in those from normal adult tissues and plasma. Proc Natl Acad Sci USA 1985;82(19):6517–21.
Loridon-Rosa B, Vielh P, Matsuura H, Clausen H, Cuadrado C, Burtin P. Distribution of oncofetal fibronectin in human mammary tumors: Immunofluorescence study on histological sections. Cancer Res 1990;50(5):1608–12.
Wunderlich H, Reichelt O, Zermann DH, Schubert J, Berndt A, Kosmehl H. Fetal fibronectin: A new screening-marker for bladder cancer? Oncol Rep 2001;8(3):669–72.
Schor SL. Fibroblast subpopulations as accelerators of tumor progression: The role of migration stimulating factor. Exs 1995;74:273–96.
Brouty-Boye D, Raux H, Azzarone B, Tamboise A, Tamboise E, Beranger S, et al. Fetal myofibroblast-like cells isolated from post-radiation fibrosis in human breast cancer. Int J Cancer 1991;47(5):697–702.
Berndt A, Kosmehl H, Katenkamp D, Tauchmann V. Appearance of the myofibroblastic phenotype in Dupuytren’s disease is associated with a fibronectin, laminin, collagen type IV and tenascin extracellular matrix. Pathobiology 1994;62(2):55–8.
Berndt A, Kosmehl H, Mandel U, Gabler U, Luo X, Celeda D, et al. TGF beta and bFGF synthesis and localization in Dupuytren’s disease (nodular palmar fibromatosis) relative to cellular activity, myofibroblast phenotype and oncofetal variants of fibronectin. Histochem J 1995;27(12):1014–20.
Ueki N, Taguchi T, Takahashi M, Adachi M, Ohkawa T, Amuro Y, et al. Inhibition of hyaluronan synthesis by vesnarinone in cultured human myofibroblasts. Biochim Biophys Acta 2000;1495(2):160–7.
Liotta LA, Kohn EC. The microenvironment of the tumour–host interface. Nature 2001;411(6835):375–9.
Spanakis E, Brouty-Boye D. Discrimination of fibroblast subtypes by multivariate analysis of gene expression. Int J Cancer 1997;71(3):402–9.
Ramaswamy A, Moll R, Barth PJ. CD34$+$ fibrocytes in tubular carcinomas and radial scars of the breast. Virchows Arch 2003;443(4):536–40.
Leygue E, Snell L, Dotzlaw H, Troup S, Hiller-Hitchcock T, Murphy LC, et al. Lumican and decorin are differentially expressed in human breast carcinoma. J Pathol 2000;192(3):313–20.
Schor SL, Ellis IR, Jones SJ, Baillie R, Seneviratne K, Clausen J, et al. Migration-stimulating Factor: A genetically truncated onco-fetal fibronectin isoform expressed by carcinoma and tumor-associated stromal cells. Cancer Res 2003;63(24):8827–36.
Schor SL, Schor AM. Phenotypic and genetic alterations in mammary stroma: Implications for tumour progression. Breast Cancer Res 2001;3(6):373–9.
Maeda T, Alexander CM, Friedl A. Induction of syndecan-1 expression in stromal fibroblasts promotes proliferation of human breast cancer cells. Cancer Res 2004;64(2):612–21.
Gudjonsson T, Ronnov-Jessen L, Villadsen R, Rank F, Bissell MJ, Petersen OW. Normal and tumor-derived myoepithelial cells differ in their ability to interact with luminal breast epithelial cells for polarity and basement membrane deposition. J Cell Sci 2002;115(Pt. 1):39–50.
Labat-Robert J. Fibronectin in malignancy: Effect of aging. Semin Cancer Biol 2002;12(3):187–95.
Silzle T, Randolph GJ, Kreutz M, Kunz-Schughart LA. The fibroblast: Sentinel cell and local immune modulator in tumor tissue. Int J Cancer 2004;108(2):173–80.
Sottile J, Hocking DC. Fibronectin polymerization regulates the composition and stability of extracellular matrix fibrils and cell-matrix adhesions. Mol Biol Cell 2002;13(10):3546–59.
Fukuda T, Yoshida N, Kataoka Y, Manabe R, Mizuno-Horikawa Y, Sato M, et al. Mice lacking the EDB segment of fibronectin develop normally but exhibit reduced cell growth and fibronectin matrix assembly in vitro. Cancer Res 2002;62(19):5603–10.
Tsunoda T, Inada H, Kalembeyi I, Imanaka-Yoshida K, Sakakibara M, Okada R, et al. Involvement of large tenascin-C splice variants in breast cancer progression. Am J Pathol 2003;162(6):1857–67.
Martin D, Brown-Luedi M, Chiquet-Ehrismann R. Tenascin-C signaling through induction of 14–3-3 tau. J Cell Biol 2003;160(2):171–5.
Bhowmick NA, Chytil A, Plieth D, Gorska AE, Dumont N, Shappell S, et al. TGF-beta signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science 2004;303(5659):848–51.
Siegel PM, Massague J. Cytostatic and apoptotic actions of TGF-beta in homeostasis and cancer. Nat Rev Cancer 2003;3(11):807–21.
Beacham DA, Cukierman E. Stromagenesis: The changing face of fibroblastic microenvironments during tumor progression. Semin Cancer Biol 2005 (in press).
Meeker AK, Hicks JL, Gabrielson E, Strauss WM, De Marzo AM, Argani P. Telomere shortening occurs in subsets of normal breast epithelium as well as in situ and invasive carcinoma. Am J Pathol 2004;164(3):925–35.
Zcharia E, Metzger S, Chajek-Shaul T, Aingorn H, Elkin M, Friedmann Y, et al. Transgenic expression of mammalian heparanase uncovers physiological functions of heparan sulfate in tissue morphogenesis, vascularization, and feeding behavior. Faseb J 2004;18(2):252–63.
Kim JB, Stein R, O’Hare MJ. Three-dimensional in vitro tissue culture models of breast cancer—A review. Breast Cancer Res Treat 2004;85(3):281–91.
Bissell MJ, Rizki A, Mian IS. Tissue architecture: The ultimate regulator of breast epithelial function. Curr Opin Cell Biol 2003;15(6):753–62.
Schmeichel KL, Bissell MJ. Modeling tissue-specific signaling and organ function in three dimensions. J Cell Sci 2003;116(12):2377–88.
Morin PJ. Drug resistance and the microenvironment: Nature and nurture. Drug Resist Updates 2003;6(4):169–72.
Hazlehurst LA, Landowski TH, Dalton WS. Role of the tumor microenvironment in mediating de novo resistance to drugs and physiological mediators of cell death. Oncogene 2003;22(47):7396–402.
Streuli CH, Bailey N, Bissell MJ. Control of mammary epithelial differentiation - basement- membrane induces tissue-specific gene-expression in the absence of cell cell-interaction and morphological polarity. J Cell Biol 1991;115(5):1383–95.
Weaver VM, Howlett AR, Langton-Webster B, Petersen OW, Bissell MJ. The development of a functionally relevant cell culture model of progressive human breast cancer. Semin Cancer Biol 1995;6(3):175–84.
Grinnell F. Fibroblast biology in three-dimensional collagen matrices. Trends Cell Biol 2003;13(5):264–9.
Yamada KM, Pankov R, Cukierman E. Dimensions and dynamics in integrin function. Braz J Med Biol Res 2003;36(8):959–66.
Cukierman E. Cell migration analyses within fibroblast-derived 3D matrices. In: Guan J, editor. Cell migration: Developmental methods and protocols. Totowa, NJ: Humana; 2004. p. 79–93.
Cukierman E. Preparation of extracellular matrices produced by cultured fibroblasts. In: Bonifacino JS, Dasso M, Lippincott-Schwartz J, Harford JB, Yamada KM, editors. Current Protocols in Cell Biology. Hoboken, NJ: Wiley; 2002. p. 10.9.1–14.
Ogmundsdottir HM, Petursdottir I, Gudmundsdottir I, Amundadottir L, Ronnov-Jessen L, Petersen OW. Effects of lymphocytes and fibroblasts on the growth of human mammary carcinoma cells studied in short-term primary cultures. In Vitro Cell Dev Biol Anim 1993;29A(12):936–42.
Beaupain R. A method for three-dimensional coculture of cancer cells combined to any other type of cells maintained organotypically. Methods Cell Sci 1999;21(1):25–30.
Seidl P, Huettinger R, Knuechel R, Kunz-Schughart LA. Three-dimensional fibroblast-tumor cell interaction causes downregulation of RACK1 mRNA expression in breast cancer cells in vitro. Int J Cancer 2002;102(2):129–36.
Shao ZM, Nguyen M, Barsky SH. Human breast carcinoma desmoplasia is PDGF initiated. Oncogene 2000;19(38):4337–45.
Tahtis K, Lee F-T, Wheatley JM, Garin-Chesa P, Park JE, Smyth FE, et al. Expression and targeting of human fibroblast activation protein in a human skin/severe combined immunodeficient mouse breast cancer Xenograft model. Mol Cancer Ther 2003;2(8):729–37.
Brown E, McKee T, DiTomaso E, Pluen A, Seed B, Boucher Y, et al. Dynamic imaging of collagen and its modulation in tumors in vivo using second-harmonic generation. Nat Med 2003;9(6):796–801.
Stoller P, Reiser KM, Celliers PM, Rubenchik AM. Polarization-modulated second harmonic generation in collagen. Biophys J 2002;82(6):3330–42.
Zoumi A, Yeh A, Tromberg BJ. Imaging cells and extracellular matrix in vivo by using second-harmonic generation and two-photon excited fluorescence. PNAS 2002;99(17):11014–19.
Yang M, Li L, Jiang P, Moossa AR, Penman S, Hoffman RM. Dual-color fluorescence imaging distinguishes tumor cells from induced host angiogenic vessels and stromal cells. Proc Natl Acad Sci USA 2003;100(24):14259–62.
Niggemann B, Drell I, Theodore L., Joseph J, Weidt C, Lang K, Zaenker KS, et al. Tumor cell locomotion: Differential dynamics of spontaneous and induced migration in a 3D collagen matrix. Exp Cell Res 2004;298(1):178–87.
Deryugina EI, Ratnikov BI, Strongin AY. Prinomastat, a hydroxamate inhibitor of matrix metalloproteinases, has a complex effect on migration of breast carcinoma cells. Int J Cancer 2003;104(5):533–41.
Friedl P, Noble PB, Walton PA, Laird DW, Chauvin PJ, Tabah RJ, et al. Migration of coordinated cell clusters in mesenchymal and epithelial cancer explants in vitro. Cancer Res 1995;55(20):4557–60.
Friedl P, Brocker EB. The biology of cell locomotion within three-dimensional extracellular matrix. Cell Mol Life Sci 2000;57(1):41–64.
Friedl P, Brocker EB. T cell migration in three-dimensional extracellular matrix: Guidance by polarity and sensations. Dev Immunol 2000;7(2–4):249–66.
Friedl P, Wolf K. Tumour-cell invasion and migration: Diversity and escape mechanisms. Nat Rev Cancer 2003;3(5):362–74.
Sahai E, Marshall CJ. Differing modes of tumour cell invasion have distinct requirements for Rho/ROCK signalling and extracellular proteolysis. Nat Cell Biol 2003;5(8):711–9.
Wolf K, Mazo I, Leung H, Engelke K, von Andrian UH, Deryugina EI, et al. Compensation mechanism in tumor cell migration: Mesenchymal-amoeboid transition after blocking of pericellular proteolysis. J Cell Biol 2003;160(2):267–77.
Geiger B. Cell biology: Encounters in Space. Science 2001;294(5547):1661–63.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cukierman, E. A Visual-Quantitative Analysis of Fibroblastic Stromagenesis in Breast Cancer Progression. J Mammary Gland Biol Neoplasia 9, 311–324 (2004). https://doi.org/10.1007/s10911-004-1403-y
Issue Date:
DOI: https://doi.org/10.1007/s10911-004-1403-y