Abstract
Among mammals, Carnivora presents an ideal group for investigating the complex interplay between functional adaptation and phylogenetic history. Here we explore mandibular form and its relationship to ecology and phylogeny using geometric morphometrics applied to mandibles of extant Carnivora. Both mandibular size and shape discriminate carnivoran ecological adaptations (diet, membership to small or large predatory guilds), but the interplay of morphology with phylogenetic history is profound. In general, families do not overlap in mandible shape; however, Viverridae, Herpestidae, Canidae, and Mustelidae exhibit functional and morphological convergence. Mandibular allometric trajectories are distinct among families and ecological categories. Our findings suggest that variability in mandibular form among Carnivora is primarily influenced by major evolutionary changes occurring at the family level and less, but significantly so, by ecological adaptations. Small generalist feeders (insectivores, omnivores) exhibit stronger convergence in mandibular shape than highly specialized predators; bigger taxa, such as bears, evolved unique morphologies constrained by allometric scaling. Thus, the findings of this study serve to demonstrate how ecological factors mold anatomical structures in similar ways to serve similar functions. As such, carnivoran species can be usefully grouped into functional ‘guilds’ in eco-morphological studies irrespective of their phylogenetic history.






Similar content being viewed by others
References
Adams DC (2008) Phylogenetic meta-analysis. Evolution 62:567–572
Adams DC, Rohlf FJ, Slice DE (2004) Geometric morphometrics: ten years of progress following the ‘revolution’. Ital J Zool 71:5–16
Andersson K, Werdelin L (2003) The evolution of cursorial carnivores in the Tertiary: implications of elbow-joint morphology. Proc R Soc B 270:S163–S165
Biknevicius AR, Van Valkenburgh B (1996) Design for killing: craniodental adaptations of mammalian predators. In: Gittleman JL (ed) Carnivore Behavior, Ecology, and Evolution Vol. 2. Cornell University Press, Ithaca, pp 393–428
Bininda-Emonds ORP, Gittleman JL (2000) Are pinnipeds functionally different from fissiped carnivores? The importance of phylogenetic comparative analyses. Evolution 54:1011–1023
Bininda-Emonds ORP, Gittleman JL, Purvis A (1999) Building large trees by combining phylogenetic information: a complete phylogeny of the extant Carnivora (Mammalia). Biol Rev Camb Philos Soc 74:143–175
Carbone C, Georgina MM, Roberts SC, Macdonald DW (1999) Energetic constraints on the diet of terrestrial carnivores. Nature 402:286–288
Carbone C, Teacher A, Rowcliffe JM (2007) The cost of carnivory. PloS Biology 5:e22
Cardini A, Tongiorgi P (2003) Yellow-bellied marmots (Marmota flaviventris) ‘in the shape space’ (Rodentia, Sciuridae): sexual dimorphism, growth and allometry of the mandible. Zoomorphology 126:121–134
Christiansen P, Adolfssen JS (2005) Bite forces, canine strength and skull allometry in carnivores (Mammmalia, Carnivora). J Zool (Lond) 266:133–151
Christiansen P, Wroe S (2007) Bite forces and evolutionary adaptations to feeding ecology in carnivores. Ecology 88:347–358
von Cramon-Taubadel N, Frazier BC, Lahr MM (2007) The problem of assessing landmark error in geometric morphometrics: theory, methods, and modifications. Am J Phys Anthropol 134:24–35
Creel S, Creel NM (2002) The African Wild Dog (Behavior, Ecology and Conservation). Princeton University Press, Princeton and Oxford
Crusafont-Pairó M, Truyols-Santonja J (1956) A biometric study of evolution of fissiped carnivores. Evolution 10:314–332
Crusafont-Pairó M, Truyols-Santonja J (1957) Estudios masterométricos en la evolución Fisípedos. I. Los módulos angulares α y β. II. Los parámetros lineales P, C, y T. Bol Inst Geol Min Esp 68:1–140
Crusafont-Pairó M, Truyols-Santonja J (1958) A quantitative study of stasigenesis in fissiped carnivores. Nature 181:289–290
Dayan T, Simberloff D (1996) Patterns of size separation in carnivore communities. In: Gittleman JL (ed) Carnivore Behavior, Ecology, and Evolution, vol 2. Cornell University Press, Ithaca, pp 243–266
Dryden IL, Mardia KV (1998) Statistical Shape Analysis. John Wiley and Sons, New York
Eisenberg JF (1981) The Mammalian Radiations. University of Chicago Press, Chicago
Evans AR, Wilson GP, Fortelius M, Jernvall J (2007) High-level similarity of dentitions in carnivorans and rodents. Nature 445:78–81
Ewer RF (1973) The Carnivores. Cornell University Press, New York
Farlow JO, Pianka ER (2002) Body size overlap, habitat partitioning and living space requirements of terrestrial vertebrate predators: implications for the paleoecology of large theropod dinosaurs. Hist Biol 16:21–40
Felsenstein J (1985) Phylogenies and the comparative method. Am Nat 125:1–15
Ford SL, Hoffman RS (1988) Potos flavus. Mammalian Species No. 321:1–9
Friscia AR, Van Valkenburgh B, Biknevicius AR (2006) An ecomorphological analysis of extant small carnivorans. J Zool (Lond) 272:82–100
Garland T, Bennett AF, Rezende EL (2005) Phylogenetic approaches in comparative physiology. J Exp Biol 208:3015–3035
Gittleman JL (1985) Carnivore body size: ecological and taxonomic correlates. Oecologia 67:540–554
Gittleman JL (1986) Carnivore brain size, behavioral ecology and phylogeny. J Mammal 67:23–36
Gittleman JL, Van Valkenburgh B (1997) Sexual size dimorphism in the canines and skulls of carnivores: effects of size, phylogeny, and behavioural ecology. J Zool (Lond) 242:97–117
Lawing AM, Polly PD (2009) Geometric morphometrics: recent applications to the study of evolution and development. J Zool (Lond) 280:1–7
Lewis ME (1997) Carnivoran paleoguilds of Africa: implications for hominid food procurement strategies. J Hum Evol 32:257–288
Macdonald DW (1992) The Velvet Claw. BBC Books, London
Maddison WP, Maddison DR (2009) Mesquite: a modular system for evolutionary analysis. Version 2.6 http://mesquiteproject.org
Meiri S, Dayan T, Simberloff D (2005a) Variability and correlations in carnivore crania and dentition. Funct Ecol 19:337–343
Meiri S, Simberloff D, Dayan T (2005b) Insular carnivore biogeography: island area and mammalian optimal body size. Am Nat 165:505–514
Meloro C, Raia P (2010) Cats and dogs down the tree: the tempo and mode of evolution in the lower carnassial of fossil and living Carnivora. Evol Biol 37:177–186
Meloro C, Raia P, Barbera C (2007) Effect of predation on prey abundance and survival in Plio-Pleistocene mammalian communities. Evol Ecol Res 9:505–525
Meloro C, Raia P, Piras P, Barbera C, O’Higgins P (2008) The shape of the mandibular corpus in large fissiped carnivores: allometry, function and phylogeny. Zool J Linn Soc 154:832–845
Miles DB, Dunham AE (1993) Historical perspectives in ecology and evolutionary biology: the use of phylogenetic comparative analyses. Annu Rev Ecol Syst 24:587–619
Monteiro LR (1999) Multivariate regression models and geometric morphometrics: the search for causal factors in the analysis of shape. Syst Biol 48:192–199
Nowak RM (1991) Walker’s Mammals of the World, 5th edn. Johns Hopkins University Press, Baltimore
O’Higgins P (2000a) The study of morphological variation in the hominid fossil record: biology, landmarks and geometry. J Anat 197:103–120
O’Higgins P (2000b) Quantitative approaches to the study of craniofacial growth and evolution: advances in morphometric techniques. In: O’Higgins P, Cohn M (eds) Vertebrate Ontogeny and Phylogeny: Implications for the Study of Hominid Skeletal Evolution. Academic Press, San Diego, pp 163–185
O’Higgins P, Jones N (1998) Facial growth in Cercocebus torquatus: an application of three dimensional geometric morphometric techniques to the study of morphological variation. J Anat 193:251–272
Polly PD (2008) Adaptive zones and the pinniped ankle: a 3D quantitative analysis of carnivoran tarsal evolution. In: Sargis E, Dagosto M (eds) Mammalian Evolutionary Morphology: A Tribute to Frederick S. Szalay. Springer, Dordrecht, pp 165–194
Polly PD, MacLeod DN (2008) Locomotion in fossil Carnivora: an application of eigensurface analysis for morphometric comparison of 3D surface. Palaeontol Electron 11, 10A: 1-13
Radinsky LB (1981a) Evolution of skull shape in carnivores, 1: representative modern carnivores. Biol J Linn Soc 15:369–388
Radinsky LB (1981b) Evolution of skull shape in carnivores, 2: additional modern carnivores. Biol J Linn Soc 16:337–355
Radinsky LB (1982) Evolution of skull shape in carnivores 3. The origin and early radiation of modern carnivores families. Paleobiology 8:177–195
Radloff FGT, Du Toit JT (2004) Large predators and their prey in a southern African savanna: a predator’s size determines its prey size range. J Anim Ecol 73:410–423
Raia P (2004) Morphological correlates of tough food consumption in carnivores. Ital J Zool 71:45–50
Raia P, Meloro C, Barbera C (2007) Incostancy in predator/prey ratio in Quaternary large mammal communities of Italy, with an appraisal of mechanisms. Quatern Res 67:255–263
Ray JC, Sunquist ME (2001) Trophic relations in a community of African rainforest carnivores. Oecologia 127:395–408
Rohlf FJ (2001) Comparative methods for the analysis of continuous variables: geometric interpretations. Evolution 55:2143–2160
Rohlf FJ (2004) Thin-plate spline. Version 1.20. Department of Ecology and Evolution, State University of New York, Stony Brook
Rohlf FJ (2006a) tpsDig 2.10. Department of Ecology and Evolution. State University of New York, Stony Brook
Rohlf FJ (2006b) tpsRelw v. 1.44. Department of Ecology and Evolution. State University of New York, Stony Brook
Rohlf FJ (2006c) NTSYSpc v. 2.21c. Exeter Software, New York
Rohlf FJ (2006d) A comment on phylogenetic correction. Evolution 60:1509–1515
Root RB (1967) The niche exploitation pattern of the Blue-gray Gnatcatcher. Ecol Monogr 37:317–350
Scapino RC (1976) Function of the digastric muscle in carnivores. J Morphol 150:843–860
Schaller GB (1972) The Serengeti Lion. (A Study of Predator-Prey Relations). The University of Chicago Press, Chicago and London
Slater JG, Dumont ER, Van Valkenburgh B (2009) Implications of predatory specialization for cranial form and function in canids. J Zool (Lond) 278:181–188
Slice DE (2007) Geometric morphometrics. Annu Rev Anthropol 36:261–81
Therrien F (2005a) Mandibular force profiles of extant carnivorans and implications for the feeding behaviour of extinct predators. J Zool (Lond) 267:249–270
Therrien F (2005b) Feeding behaviour and bite force of sabretoothed predators. Zool J Linn Soc 145:393–426
Van Valkenburgh B (1985) Locomotor diversity between past and present guilds of large predatory mammals. Paleobiology 11:406–428
Van Valkenburgh B (1988) Trophic diversity in past and present guilds of large predatory mammals. Paleobiology 14:155–173
Van Valkenburgh B (1989) Carnivore dental adaptations and diet: a study of trophic diversity within guilds. In: Gittleman JL (ed) Carnivore Behavior, Ecology, and Evolution Vol. 1. Cornell University Press, Ithaca, pp 410–436
Van Valkenburgh B (1996) Feeding behaviour in free-ranging, large African carnivore. J Mammal 77:240–254
Van Valkenburgh B (2007) Déjà vu: the evolution of feeding morphologies in the Carnivora. Integr Comp Biol 1—17
Van Valkenburgh B, Janis CM (1993) Historical diversity patterns in North American large herbivores and carnivores. In: Ricklefs RE, Schluter D (eds) Species diversity in ecological communities. The University of Chicago Press, Chicago, pp 330–340
Van Valkenburgh B, Wayne RK (1994) Shape divergence associated with size convergence in sympatric East Africa jackals. Ecology 75:1567–1581
Vezina AF (1985) Empirical relationship between predator and prey size among terrestrial vertebrate predators. Oecologia 67:555–565
Werdelin L (1989) Constraint and adaptation in the bonecracking canid Osteoborus (Mammalia: Canidae). Paleobiology 15:387–401
Werdelin L (1996) Carnivoran ecomorphology: a phylogenetic perspective. In: Gittleman JL (ed) Carnivore Behavior, Ecology, and Evolution. Vol. 1. Cornell University Press, Ithaca, pp 582–624
Werdelin L, Solounias N (1991) The Hyaenidae: taxonomy, systematic and evolution. Fossils and Strata 30:1–104
Wesley-Hunt GD (2005) The morphological diversification of carnivores in North America. Paleobiology 31:35–55
Woodroffe R, Ginsberg JR (2005) King of the beasts? Evidence for guild redundancy among large mammalian carnivores. In: Ray JC, Redford KH, Steneck RS, Berger J (eds) Large Carnivores and the Conservation of Biodiversity. Island Press, Washington, pp 230–246
Zelditch ML, Swiderski DL, Sheets HD, Fink WL (2004) Geometric Morphometrics for Biologists. A Primer. Elsevier, San Diego
Acknowledgements
We are grateful to the staff of the Natural History Museum, London for their invaluable help. In particular, we would like to thank: P. Jenkins, L. Tomsett, R. Portela-Miguez, A. Salvador, and D. Hills. A. Kitchner and J. Herman provided support in the collections of the Royal Museum of Scotland. P. Piras and F. Lucci kindly shared their mandible database of felids. P. Raia, F. Carotenuto, C. Barbera, and T. Kotsakis helped throughout the preliminary analyses for this manuscript. John Wible and two anonymous reviewers kindly provided important insights to improve the quality of the manuscript
Author information
Authors and Affiliations
Corresponding author
Appendices
Appendix 1
Appendix 2
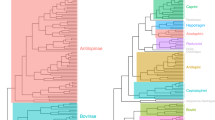
Phylogenetic tree of 97 Carnivora after pruning the supertree of Bininda-Emonds et al. (1999) to only include the taxa studied here. Branch lengths are proportional to time of divergence in millions of years.

Rights and permissions
About this article
Cite this article
Meloro, C., O’Higgins, P. Ecological Adaptations of Mandibular Form in Fissiped Carnivora. J Mammal Evol 18, 185–200 (2011). https://doi.org/10.1007/s10914-011-9156-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10914-011-9156-z