Abstract
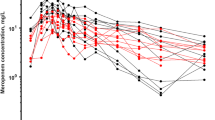
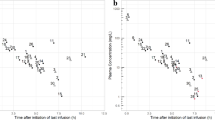
The objective was to demonstrate the methodology and process of optimal sparse sampling pharmacokinetics (PK). This utilized a single daily dose of pioglitazone for pediatric patients with severe sepsis and septic shock based upon adult and minimal adolescent data. Pioglitazone pharmacokinetics were modeled using non-compartment analysis WinNonlin Pro (version 5.1) and population kinetics using NONMEM (version 7.1) with first order conditional estimation method (FOCE) with interaction. The initial model was generated from single- and multiple-dose pioglitazone PK data (15 mg, 30 mg, and 45 mg) in 36 adolescents with diabetes. PK models were simulated and overlaid upon original data to provide a comparison best described by a single compartment, first order model. The optimal design was based on the simulated oral administration of pioglitazone to three groups of pediatric patients, age 3.8 (2–6 years), weight 14.4 (7–28 kg); age 9.6 (6.1–11.9 years), weight 36.5 (28.1–48 kg) and age 15.5 (12–17 years,) weight 61.6 (48.1–80 kg). PFIM (version 3.2) was used to evaluate sample study size. Datasets were compiled using simulation for each dose (15, 30 and 45 mg) for the potential age/weight groups. A target dose of 15 mg daily in the youngest and middle groups was considered appropriate with area under the curve exposure levels (AUC) comparable to studies in adolescents. The final optimal design suggested time points of 0.5, 2, 6 and 21 h for 24 h dosing. This methodology provides a robust method of utilizing adult and limited adolescent data to simulate allometrically scaled, pediatric data sets that allow the optimal design of a pediatric trial. The pharmacokinetics of pioglitazone were described adequately and simulated data estimates were comparable to literature values. The optimal design provided clinically attainable sample times and windows.






Similar content being viewed by others
References
Eckland DA, Danhof M (2000) Clinical pharmacokinetics of pioglitazone. Exp Clin Endocrinol Diabetes 108:234–242
Jiang C, Ting AT, Seed B (1998) PPAR-gamma agonists inhibit production of monocyte inflammatory cytokines. Nature 391:82–86
Zingarelli B, Sheehan M, Hake PW, O’Connor M, Denenberg A, Cook JA (2003) Peroxisome proliferator activator receptor-gamma ligands, 15-deoxy-Delta(12, 14)-prostaglandin J2 and ciglitazone, reduce systemic inflammation in polymicrobial sepsis by modulation of signal transduction pathways. J Immunol 171:6827–6837
Collin M, Patel NS, Dugo L, Thiemermann C (2004) Role of peroxisome proliferator-activated receptor-gamma in the protection afforded by 15-deoxydelta12, 14 prostaglandin J2 against the multiple organ failure caused by endotoxin. Crit Care Med 32:826–831
Kaplan JM, Cook JA, Hake PW, O’Connor M, Burroughs TJ, Zingarelli B (2005) 15-deoxy-delta12, 14-prostaglandin J2 (15D-PGJ2), a peroxisome proliferator activated receptor gamma ligand, reduces tissue leukosequestration and mortality in endotoxic shock. Shock 24:59–65
Kuboki S, Shin T, Huber N, Eismann T, Galloway E, Schuster R, Blanchard J, Zingarelli B, Lentsch AB (2008) Peroxisome proliferator-activated receptor-gamma protects against hepatic ischemia/reperfusion injury in mice. Hepatology 47:215–224
Zingarelli B, Hake PW, Mangeshkar P, O’Connor M, Burroughs TJ, Piraino G, Denenberg A, Wong HR (2007) Diverse cardioprotective signaling mechanisms of peroxisome proliferator-activated receptor-gamma ligands, 15-deoxy-Delta12, 14-prostaglandin J2 and ciglitazone, in reperfusion injury: role of nuclear factor-kappaB, heat shock factor 1, and Akt. Shock 28:554–563
Chima RS, Hake PW, Piraino G, Mangeshkar P, Denenberg A, Zingarelli B (2008) Ciglitazone ameliorates lung inflammation by modulating the inhibitor kappaB protein kinase/nuclear factor-kappaB pathway after hemorrhagic shock. Crit Care Med 36:2849–2857
Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR (2001) Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med 29:1303–1310
Watson RS, Carcillo JA, Linde-Zwirble WT, Clermont G, Lidicker J, Angus DC (2003) The epidemiology of severe sepsis in children in the United States. Am J Respir Crit Care Med 167:695–701
Bone RC, Sibbald WJ, Sprung CL (1992) The ACCP-SCCM consensus conference on sepsis and organ failure. Chest 101:1481–1483
Christensen ML, Meibohm B, Capparelli EV, Velasquez-Mieyer P, Burghen GA, Tamborlane WV (2005) Single- and multiple-dose pharmacokinetics of pioglitazone in adolescents with type 2 diabetes. J Clin Pharmacol 45:1137–1144
Budde K, Neumayer HH, Fritsche L, Sulowicz W, Stompor T, Eckland D (2003) The pharmacokinetics of pioglitazone in patients with impaired renal function. Br J Clin Pharmacol 55:368–374
Anderson BJ, Holford NH (2008) Mechanism-based concepts of size and maturity in pharmacokinetics. Annu Rev Pharmacol Toxicol 48:303–332
FDA (1998) Guidance for industry general considerations for pediatric pharmacokientics studies for drugs and biological products. US Department of Health and Human Services; Food and Drug Adminstration; Centre for Drug Evaluation and Research & Centre for Biologics Evaluation and Research, Rockville (Accessed 10 July, 2010)
Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, Wei R, Curtin LR, Roche AF, Johnson CL (2002) 2000 CDC Growth Charts for the United States: methods and development. Vital Health Stat 11:1–190
Anderson BJ, Allegaert K, Holford NH (2006) Population clinical pharmacology of children: modelling covariate effects. Eur J Pediatr 165:819–829
Kearns GL, Abdel-Rahman SM, Alander SW, Blowey DL, Leeder JS, Kauffman RE (2003) Developmental pharmacology—drug disposition, action, and therapy in infants and children. N Engl J Med 349:1157–1167
Anderson BJ, Allegaert K, Holford NH (2006) Population clinical pharmacology of children: general principles. Eur J Pediatr 165:741–746
Siegel JH, Cerra FB, Coleman B, Giovannini I, Shetye M, Border JR, McMenamy RH (1979) Physiological and metabolic correlations in human sepsis. Invited commentary. Surgery 86:163–193
De Paepe P, Belpaire FM, Buylaert WA (2002) Pharmacokinetic and pharmacodynamic considerations when treating patients with sepsis and septic shock. Clin Pharmacokinet 41:1135–1151
Duffull S, Denman NG, Eccleston J, Kimko HC. WinPOPT. School of Pharmacy, University of Otago, Dunedin, New Zealand; Available from: http://www.winpopt.com/
Kimko HC, Duffull SB (2003) Simulation for designing clinical trials: a pharmacokinetic-pharmacodynamic modeling perspective. Marcel Dekker, New York
Waterhouse TH, Redmann S, Duffull SB, Eccleston JA (2005) Optimal design for model discrimination and parameter estimation for itraconazole population pharmacokinetics in cystic fibrosis patients. J Pharmacokinet Pharmacodyn 32:521–545
Duffull S, Waterhouse T, Eccleston J (2005) Some considerations on the design of population pharmacokinetic studies. J Pharmacokinet Pharmacodyn 32:441–457
Bazzoli C, Retout S, Mentre F (2009) Design evaluation and optimisation in multiple response nonlinear mixed effect models: PFIM 3.0. Comput Methods Programs Biomed 98:55–65
Gobburu J (2010) How to double success rate of pediatric trials? Conference proceedings of ASCPT, Atlanta, GA
Acknowledgments
The authors would like to acknowledge financial support from the following NIH grant 5T32AR007594-15 (CMTS), 1K24HD050387-04 (AV) and K08GM093135-01 (JK). The authors would like to thank and acknowledge the authors from the publication by Christensen et al. [12] for sharing their data.
Conflict of interest
The authors have no conflicts of interest that are directly relevant to the content of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sherwin, C.M.T., Ding, L., Kaplan, J. et al. Optimal study design for pioglitazone in septic pediatric patients. J Pharmacokinet Pharmacodyn 38, 433–447 (2011). https://doi.org/10.1007/s10928-011-9202-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10928-011-9202-8