Abstract
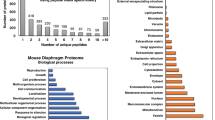
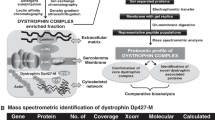
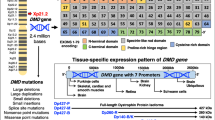
Progressive x-linked muscular dystrophy represents the most commonly inherited neuromuscular disorder in humans. Although the disintegration of the dystrophin-associated glycoprotein complex triggers the initial pathogenesis of Duchenne muscular dystrophy, secondary alterations in metabolic pathways, cellular signaling and the regulation of ion homeostasis are probably crucial factors that cause end-stage fibre degeneration. The application of mass spectrometry-based proteomics for the global cataloguing of muscle biomarkers has recently been applied to the analysis of the mdx animal model of muscular dystrophy and the biochemical evaluation of experimental exon skipping therapy. The fluorescence difference in-gel electrophoretic analysis of normal versus mdx diaphragm muscle revealed changed expression levels of proteins involved in nucleotide metabolism, Ca2+-handling, the cellular stress response and key bioenergetic processes. The swift up-regulation of small heat shock proteins, such as cvHsp, seems to form an integral part of the repair mechanisms in dystrophic fibres and may be exploitable as a new option to treat inherited muscle degeneration. Importantly, the mass spectrometry-based profiling of mdx muscle following the specific removal of exon 23 in the mutated dystrophin gene transcript showed a partial reversal of important secondary changes. Experimental exon skipping restored the expression of the dystrophin isoform Dp427, its associated glycoprotein β-dystroglycan, neuronal nitric oxide synthase, calsequestrin, adenylate kinase and the muscle-specific stress protein cvHsp. In the future, a well-defined set of signature molecules could be used to improve diagnosis, monitor disease progression, identify new therapeutic pathways, and validate the effects of novel drugs or experimental treatments such as gene therapy.






Similar content being viewed by others
References
Ahn AH, Kunkel LM (1993) The structural and functional diversity of dystrophin. Nat Genet 3:283–291
Alagaratnam S, Mertens BJ, Dalebout JC, Deelder AM, van Ommen GJ, den Dunnen JT, ‘t Hoen PA (2008) Serum protein profiling in mice: identification of factor XIIIa as a potential biomarker for muscular dystrophy. Proteomics 8:1552–1563
Alderton JM, Steinhardt RA (2000) Calcium influx through calcium leak channels is responsible for the elevated levels of calcium-dependent proteolysis in dystrophic myotubes. J Biol Chem 275:9452–9460
Allamand V, Campbell KP (2000) Animal models for muscular dystrophy: valuable tools for the development of therapies. Hum Mol Genet 9:2459–2467
Alter J, Lou F, Rabinowitz A, Yin H, Rosenfeld J, Wilton SD, Partridge TA, Lu QL (2006) Systemic delivery of morpholino oligonucleotide restores dystrophin expression bodywide and improves dystrophic pathology. Nat Med 12:175–177
Anderson NL, Anderson NG (2002) The human plasma proteome: history, character, and diagnostic prospects. Mol Cell Proteomics 1:845–867
Baker PE, Kearney JA, Gong B, Merriam AP, Kuhn DE, Porter JD, Rafael-Fortney JA (2006) Analysis of gene expression differences between utrophin/dystrophin-deficient vs mdx skeletal muscles reveals a specific upregulation of slow muscle genes in limb muscles. Neurogenetics 7:81–91
Bellinger AM, Reiken S, Carlson C, Mongillo M, Liu X, Rothman L, Matecki S, Lacampagne A, Marks AR (2009) Hypernitrosylated ryanodine receptor calcium release channels are leaky in dystrophic muscle. Nat Med 15:325–330
Borman L, Polla BS, Lotz BP, Gericke GS (1995) Expression of heat shock/stress proteins in Duchenne muscular dystrophy. Muscle Nerve 18:23–31
Bouchentouf M, Benabdallah BF, Tremblay JP (2004) Myoblast survival enhancement and transplantation success improvement by heat-shock treatment in mdx mice. Transplantation 77:1349–1356
Campbell KP (1995) Three muscular dystrophies: loss of cytoskeleton-extracellular matrix linkage. Cell 80:675–679
Canas B, Lopez-Ferrer D, Ramos-Fernandez A, Camafeita E, Calvo E (2006) Mass spectrometry technologies for proteomics. Brief Funct Genomic Proteomic 4:295–320
Capitanio D, Vasso M, Fania C, Moriggi M, Vigano A, Procacci P, Magnaghi V, Gelfi C (2009) Comparative proteomic profile of rat sciatic nerve and gastrocnemius muscle tissues in ageing by 2-D DIGE. Proteomics 9:2004–2020
Chen YW, Zhao P, Borup R, Hoffman EP (2000) Expression profiling in the muscular dystrophies: identification of novel aspects of molecular pathophysiology. J Cell Biol 151:1321–1336
Cox GF, Kunkel LM (1997) Dystrophies and heart disease. Curr Opin Cardiol 12:329–343
Cox J, Mann M (2007) Is proteomics the new genomics? Cell 130:395–398
Cullen MJ, Walsh J, Nicholson LV, Harris JB (1990) Ultrastructural localization of dystrophin in human muscle by using gold immunolabelling. Proc R Soc Lond B Biol Sci 240:197–210
Culligan K, Banville N, Dowling P, Ohlendieck K (2002) Drastic reduction of calsequestrin-like proteins and impaired calcium binding in dystrophic mdx muscle. J Appl Physiol 92:435–445
de Hoog CL, Mann M (2004) Proteomics. Annu Rev Genomics Hum Genet 5:267–293
de Jong WW, Leunissen JA, Voorter CE (1993) Evolution of the alpha-crystallin/small heat shock protein family. Mol Biol Evol 10:103–126
DiFranco M, Woods CE, Capote J, Vergara JL (2008) Dystrophic skeletal muscle fibers display alterations at the level of calcium microdomains. Proc Natl Acad Sci USA 105:14698–14703
Divet A, Huchet-Cadiou C (2002) Sarcoplasmic reticulum function in slow- and fast-twitch skeletal muscles from mdx mice. Pflugers Arch 444:634–643
Domon B, Aebersold R (2006) Mass spectrometry and protein analysis. Science 312:212–217
Doran P, Dowling P, Lohan J, McDonnell K, Poetsch S, Ohlendieck K (2004) Subproteomics analysis of Ca2+-binding proteins demonstrates decreased calsequestrin expression in dystrophic mouse skeletal muscle. Eur J Biochem 271:3943–3952
Doran P, Martin G, Dowling P, Jockusch H, Ohlendieck K (2006a) Proteome analysis of the dystrophin-deficient MDX diaphragm reveals a drastic increase in the heat shock protein cvHsp. Proteomics 6:4610–4621
Doran P, Dowling P, Donoghue P, Buffini M, Ohlendieck K (2006b) Reduced expression of regucalcin in young and aged mdx diaphragm indicates abnormal cytosolic calcium handling in dystrophin-deficient muscle. Biochim Biophys Acta 1764:773–785
Doran P, Gannon J, O’Connell K, Ohlendieck K (2007) Proteomic profiling of animal models mimicking skeletal muscle disorders. Proteomics Clin Appl 1:1169–1184
Doran P, Donoghue P, O’Connell K, Gannon J, Ohlendieck K (2009a) Proteomics of skeletal muscle aging. Proteomics 9:989–1003
Doran P, Wilton SD, Fletcher S, Ohlendieck K (2009b) Proteomic profiling of antisense-induced exon skipping reveals reversal of pathobiochemical abnormalities in dystrophic mdx diaphragm. Proteomics 9:671–685
Dowling P, Culligan K, Ohlendieck K (2002) Distal mdx muscle groups exhibiting up-regulation of utrophin and rescue of dystrophin-associated glycoproteins exemplify a protected phenotype in muscular dystrophy. Naturwissenschaften 89:75–88
Dowling P, Lohan J, Ohlendieck K (2003) Comparative analysis of Dp427-deficient mdx tissues shows that the milder dystrophic phenotype of extraocular and toe muscle fibres is associated with a persistent expression of beta-dystroglycan. Eur J Cell Biol 82:222–230
Dowling P, Doran P, Ohlendieck K (2004) Drastic reduction of sarcalumenin in Dp427 (dystrophin of 427 kDa)-deficient fibres indicates that abnormal calcium handling plays a key role in muscular dystrophy. Biochem J 379:479–488
Durbeej M, Campbell KP (2002) Muscular dystrophies involving the dystrophin-glycoprotein complex: an overview of current mouse models. Curr Opin Genet Dev 12:349–361
Emery AE (2002a) The muscular dystrophies. Lancet 359:687–695
Emery AE (2002b) Muscular dystrophy into the new millennium. Neuromuscul Disord 12:343–349
Emery A, Muntoni F (2003) Duchenne muscular dystrophy, 3rd edn. Oxford University Press, Oxford
Ervasti JM, Sonnemann KJ (2008) Biology of the striated muscle dystrophin-glycoprotein complex. Int Rev Cytol 265:191–225
Ervasti JM, Ohlendieck K, Kahl SD, Gaver MG, Campbell KP (1990) Deficiency of a glycoprotein component of the dystrophin complex in dystrophic muscle. Nature 345:315–319
Fenn JB, Mann M, Meng CK, Wong SF, Whitehouse CM (1989) Electrospray ionization for mass spectrometry of large biomolecules. Science 246:64–71
Ferretti R, Marques MJ, Pertille A, Neto HS (2009) Sarcoplasmic-endoplasmic-reticulum Ca2+ -ATPase and calsequestrin are overexpressed in spared intrinsic laryngeal muscles of dystrophin-deficient mdx mice. Muscle Nerve 39:609–615
Gauthier DJ, Lazure C (2008) Complementary methods to assist subcellular fractionation in organellar proteomics. Expert Rev Proteomics 5:603–617
Ge Y, Molloy MP, Chamberlain JS, Andrews PC (2003) Proteomic analysis of mdx skeletal muscle: great reduction of adenylate kinase 1 expression and enzymatic activity. Proteomics 3:1895–1903
Ge Y, Molloy MP, Chamberlain JS, Andrews PC (2004) Differential expression of the skeletal muscle proteome in mdx mice at different ages. Electrophoresis 25:2576–2585
Gehrig SM, Ryall JG, Schertzer JD, Lynch GS (2008) Insulin-like growth factor-I analogue protects muscles of dystrophic mdx mice from contraction-mediated damage. Exp Physiol 93:1190–1198
Gervasio OL, Whitehead NP, Yeung EW, Phillips WD, Allen DG (2008) TRPC1 binds to caveolin-3 and is regulated by Src kinase—role in Duchenne muscular dystrophy. J Cell Sci 121:2246–2255
Goerg A, Weiss W, Dunn MJ (2004) Current two-dimensional electrophoresis technology for proteomics. Proteomics 4:3665–3685
Griffin JL, Des Rosiers C (2009) Applications of metabolomics and proteomics to the mdx mouse model of Duchenne muscular dystrophy: lessons from downstream of the transcriptome. Genome Med 1:32.1–32.11
Gulston MK, Rubtsov DV, Atherton HJ, Clarke K, Davies KE, Lilley KS, Griffin JL (2008) A combined metabolomic and proteomic investigation of the effects of a failure to express dystrophin in the mouse heart. J Proteome Res 7:2069–2077
Haslett JN, Sanoudou D, Kho AT, Bennett RR, Greenberg SA, Kohane IS, Beggs AH, Kunkel LM (2003) Gene expression comparison of biopsies from Duchenne muscular dystrophy (DMD) and normal skeletal muscle. Proc Natl Acad Sci USA 99:15000–15005
Henry MD, Campbell KP (1999) Dystroglycan inside and out. Curr Opin Cell Biol 11:602–607
Isfort RJ (2002) Proteomic analysis of striated muscle. J Chromatogr B 771:155–165
Kinali M, Arechavala-Gomeza V, Feng L, Cirak S, Hunt D, Adkin C, Guglieri M, Ashton E, Abbs S, Nihoyannopoulos P, Garralda ME, Rutherford M, McCulley C, Popplewell L, Graham IR, Dickson G, Wood MJ, Wells DJ, Wilton SD, Kole R, Straub V, Bushby K, Sewry C, Morgan JE, Muntoni F (2009) Local restoration of dystrophin expression with the morpholino oligomer AVI-4658 in Duchenne muscular dystrophy: a single-blind, placebo-controlled, dose-escalation, proof-of-concept study. Lancet Neurol 8:918–928
Koenig M, Kunkel LM (1990) Detailed analysis of the repeat domain of dystrophin reveals four potential hinge segments that may confer flexibility. J Biol Chem 265:4560–4566
Koenig M, Monaco AP, Kunkel LM (1988) The complete sequence of dystrophin predicts a rod-shaped cytoskeletal protein. Cell 53:219–226
Krueger J, Kunert-Keil C, Bisping F, Brinkmeier H (2008) Transient receptor potential cation channels in normal and dystrophic mdx muscle. Neuromuscul Disord 18:501–513
Lovering RM, Michaelson L, Ward CW (2009) Malformed mdx myofibers have normal cytoskeletal architecture yet altered EC coupling and stress-induced Ca2+ signaling. Am J Physiol Cell Physiol 297:C571–C580
Mallouk N, Jacquemond V, Allard B (2000) Elevated subsarcolemmal Ca2+ in mdx mouse skeletal muscle fibres detected with Ca2+-activated K+ channels. Proc Natl Acad Sci USA 97:4950–4955
Manzur AY, Muntoni F (2009) Diagnosis and new treatments in muscular dystrophies. J Neurol Neurosurg Psychiatry 80:706–714
Manzur AY, Kuntzer T, Pike M, Swan A (2008) Glucocorticoid corticosteroids for Duchenne muscular dystrophy. Cochrane Database Syst Rev (1):CD003725
Marouga R, David S, Hawkins E (2005) The development of the DIGE system: 2D fluorescence difference gel analysis technology. Anal Bioanal Chem 382:669–678
Marques MJ, Ferretti R, Vomero VU, Minatel E, Neto HS (2007) Intrinsic laryngeal muscles are spared from myonecrosis in the mdx mouse model of Duchenne muscular dystrophy. Muscle Nerve 35:349–353
Matsumura CY, Pertille A, Albuquerque TC, Santo Neto H, Marques MJ (2009) Diltiazem and verapamil protect dystrophin-deficient muscle fibers of MDX mice from degeneration: a potential role in calcium buffering and sarcolemmal stability. Muscle Nerve 39:167–176
Miller G, Wang EL, Nassar KL, Peter AK, Crosbie RH (2007) Structural and functional analysis of the sarcoglycan-sarcospan subcomplex. Exp Cell Res 313:639–651
Minden JS, Dowd SR, Meyer HE, Stuehler K (2009) Difference gel electrophoresis. Electrophoresis 30:S156–S161
Monaco AP, Neve RL, Colletti-Feener C, Bertelson CJ, Kurnit DM, Kunkel LM (1986) Isolation of candidate cDNAs for portions of the Duchenne muscular dystrophy gene. Nature 323:646–650
Muir LA, Chamberlain JS (2009) Emerging strategies for cell and gene therapy of the muscular dystrophies. Expert Rev Mol Med 11:e18
Nakamura A, Takeda S (2009) Exon-skipping therapy for Duchenne muscular dystrophy. Neuropathology 29:494–501
Nicholl ID, Quinlan RA (1994) Chaperone activity of alpha-crystallins modulates intermediate filament assembly. EMBO J 13:945–953
Ohlendieck K (1996) Towards an understanding of the dystrophin-glycoprotein complex: linkage between the extracellular matrix and the subsarcolemmal membrane cytoskeleton. Eur J Cell Biol 69:1–10
Ohlendieck K, Campbell KP (1991) Dystrophin constitutes 5% of membrane cytoskeleton in skeletal muscle. FEBS Lett 283:230–234
Ohlendieck K, Ervasti JM, Matsumura K, Kahl SD, Leveille CJ, Campbell KP (1991a) Dystrophin-related protein is localized to neuromuscular junctions of adult skeletal muscle. Neuron 7:499–508
Ohlendieck K, Ervasti JM, Snook JB, Campbell KP (1991b) Dystrophin glycoprotein complex is highly enriched in skeletal muscle sarcolemma. J Cell Biol 112:135–148
Ohlendieck K, Matsumura K, Ionasescu VV, Towbin JA, Bosch P, Weinstein SL, Sernett SW, Campbell KP (1993) Duchenne muscular dystrophy: deficiency of dystrophin-associated proteins in the sarcolemma. Neurology 43:795–800
Ozawa E, Mizuno Y, Hagiwara Y, Sasaoka T, Yoshida M (2005) Molecular and cell biology of the sarcoglycan complex. Muscle Nerve 32:563–576
Piccolo F, Moore SA, Mathews KD, Campbell KP (2002) Limb-girdle muscular dystrophies. Adv Neurol 88:273–291
Pieper R, Gatlin CL, Makusky AJ, Russo PS, Schatz CR, Miller SS, Su Q, McGrath AM, Estock MA, Parmar PP, Zhao M, Huang ST, Zhou J, Wang F, Esquer-Blasco R, Anderson NL, Taylor J, Steiner S (2003) The human serum proteome: display of nearly 3700 chromatographically separated protein spots on two-dimensional electrophoresis gels and identification of 325 distinct proteins. Proteomics 3:1345–1364
Rabilloud T, Vaezzadeh AR, Potier N, Lelong C, Leize-Wagner E, Chevallet M (2009) Power and limitations of electrophoretic separations in proteomics strategies. Mass Spectrom Rev 28:816–843
Rees ML, Lien CF, Gorecki DC (2007) Dystrobrevins in muscle and non-muscle tissues. Neuromuscul Disord 17:123–134
Sicinski P, Geng Y, Ryder-Cook AS, Barnard EA, Darlison MG, Barnard PJ (1989) The molecular basis of muscular dystrophy in the mdx mouse: a point mutation. Science 244:1578–1580
Stedman HH, Sweeney HL, Shrager JB, Maguire HC, Panettieri RA, Petrof B, Narusawa M, Leferovich JM, Sladky JT, Kelly AM (1991) The mdx mouse diaphragm reproduces the degenerative changes of Duchenne muscular dystrophy. Nature 352:536–539
Teichmann MD, Wegner FV, Fink RH, Chamberlain JS, Launikonis BS, Martinac B, Friedrich O (2008) Inhibitory control over Ca2+ sparks via mechanosensitive channels is disrupted in dystrophin deficient muscle but restored by mini-dystrophin expression. PLoS One 3:e3644
Thomas LB, Joseph GL, Adkins TD, Andrade FH, Stemple JC (2008) Laryngeal muscles are spared in the dystrophin deficient mdx mouse. J Speech Lang Hear Res 51:586–595
Torres LFB, Duchen LW (1987) The mutant mdx: inherited myopathy in the mouse. Brain 110:269–299
Turk R, Sterrenburg E, de Meijer EJ, van Ommen GJ, den Dunnen JT, ‘t Hoen PA (2005) Muscle regeneration in dystrophin-deficient mdx mice studied by gene expression profiling. BMC Genomics 6:e98
Turner PR, Schultz R, Ganguly B, Steinhardt RA (1993) Proteolysis results in altered leak channel kinetics and elevated free calcium in mdx muscle. J Membr Biol 133:243–251
van Ommen GJ, van Deutekom J, Aartsma-Rus A (2008) The therapeutic potential of antisense-mediated exon skipping. Curr Opin Mol Ther 10:140–149
Vandebrouck C, Martin D, Colson-Van Schoor M, Debaix H, Gailly P (2002) Involvement of TRPC in the abnormal calcium influx observed in dystrophic (mdx) mouse skeletal muscle fibers. J Cell Biol 158:1089–1096
Viswanathan S, Unlu M, Minden JS (2006) Two-dimensional difference gel electrophoresis. Nat Protoc 1:1351–1358
Wilton SD, Fletcher S (2008) Exon skipping and Duchenne muscular dystrophy: hope, hype and how feasible? Neurol India 56:254–262
Wittmann-Liebold B, Graack HR, Pohl T (2006) Two-dimensional gel electrophoresis as tool for proteomics studies in combination with protein identification by mass spectrometry. Proteomics 6:4688–4703
Woods CE, Novo D, DiFranco M, Vergara JL (2004) The action potential-evoked sarcoplasmic reticulum calcium release is impaired in mdx mouse muscle fibres. J Physiol 557:59–75
Zaluzec EJ, Gage DA, Watson JT (1995) Matrix-assisted laser desorption ionization mass spectrometry: applications in peptide and protein characterization. Protein Expr Purif 6:109–123
Zhang SZ, Xie HQ, Xu Y, Li XQ, Wei RQ, Zhi W, Deng L, Qiu L, Yang ZM (2008) Regulation of cell proliferation by fast myosin light chain 1 in myoblasts derived from extraocular muscle, diaphragm and gastrocnemius. Exp Biol Med 233:1374–1384
Acknowledgments
The authors thank Muscular Dystrophy Ireland, the Irish Health Research Board, the Higher Education Authority and Science Foundation Ireland for continued support of our muscle research programme. We would like to thank past and present collaborators and members of the NUIM Muscle Biology Laboratory for all their help and encouragement to establish a muscle proteomics unit in NUI Maynooth over the last few years.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lewis, C., Carberry, S. & Ohlendieck, K. Proteomic profiling of x-linked muscular dystrophy. J Muscle Res Cell Motil 30, 267–279 (2009). https://doi.org/10.1007/s10974-009-9197-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10974-009-9197-6