Abstract
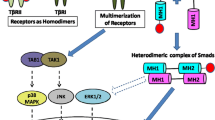
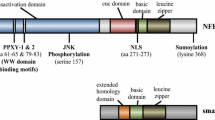
Tsc-22 was isolated as a TGF-β-inducible gene by differential screening of the mouse osteoblastic cell cDNA library [J Biol Chem 267 (1992) 10219]. tsc-22 mRNA is expressed in almost all organs of mice and humans and its expression is induced in a variety of cell lines by many different factors including TGF-β, phorbol ester, serum, and progestin. tsc-22 encodes a 18-kd protein that contains a leucine zipper motif and a Tsc-box. The leucine zipper motif of the Tsc-22 protein does not have a basic DNA binding motif and when the protein was fused to a heterologous DNA binding domain, it showed various transcription-modulating activities ranging from activation to repression [J Biol Chem 274 (1999) 27439, Biochem Biophys Res Commun 278 (2000) 659]. Although these results suggest that the Tsc-22 protein functions as a transcriptional regulator recruiting various coactivators or repressors, its mechanism is not known. In this study, we examined whether Tsc-22 modulates the TGF-β-dependant signaling pathway and found that Tsc-22 binds to and modulate the transcriptional activity of Smad3 and Smad4. Its effect on cellular differentiation was also examined. (Mol Cell Biochem 271: 23–28, 2005)
Similar content being viewed by others
References
Shibanuma M, Kuroki T, Nose K: Isolation of a gene encoding a putative leucine zipper structure that is induced by transforming growth factor beta 1 and other growth factors. J Biol Chem 267: 10219–10224, 1992
Kester HA, Blanchetot C, den Hertog J, van der Saag PT, van der Burg B: Transforming growth factor-beta-stimulated clone-22 is a member of a family of leucine-zipper proteins that can homodimerize and heterodimerize and has transcriptional repressor activity. J Biol Chem 274: 27439–27447, 1999
Hino S, Kawamata H, Uchida D, Omotehara F, Miwa Y, Begum NM, Yoshida H, Fujimori T, Sato M: Nuclear translocation of Tsc-22 concomitant with apoptosis: Tsc-22 as a putative transcriptional regulator. Biochem Biophys Res Commun 278: 659–664, 2000
Massague J, Wotton D: Transcriptional control by the TGF-β/Smad signaling system. EMBO J 19: 1745–1754, 2000
Itoh S, Itoh F, Goumans M, ten Dijke P: Signaling of transforming growth factor-β family members through Smad proteins. Eur J Biochem 267: 6954–6967, 2000
Oft M, Peli J, Rudaz C, Schwartz H, Beug H, Reichmann E: Tgf-beta1 and Ha-Ras collaborate in modulating the phenotypic plasticity and invasiveness of epithelial tumors. Genes Dev 10: 2462–2477, 1996
Feng XH, Zhang Y, Wu RY, Derynck R: The tumor suppressor Smad4/DPC4 and transcriptional adaptor CBP/p300 are coactivators for smad3 in TGF-beta-induced transcriptional activation. Genes Dev 12: 2153–2163, 1998
Janknecht R, Wells NJ, Hunter T: TGF-beta-stimulated cooperation of smad proteins with the coactivators CBP/p300. Genes Dev 12: 2144–2119, 1998
Nishihara A, Hanai J, Okamoto N, Yanagisawa J, Kato S, Miyazono K, Kawabata M: Role of p300, a transcriptional coactivator, in signalling of TGF-beta. Genes Cells 3: 613–623, 1998
Pouponnot C, Jayaraman L, Massague J: Physical and functional interaction of SMADs and p300/CBP. J Biol Chem 273: 22865–22868, 1998
Shen X, Hu PP, Liberati NT, Datto MB, Frederick JP, Wang XF: TGF-beta-induced phosphorylation of Smad3 regulates its interaction with coactivator p300/CREB-binding protein. Mol Biol Cell 9: 3309–3319, 1998
Topper JN, DiChiara MR, Brown JD, Williams AJ, Falb D, Collins T, Gimbrone MA, Jr: CREB binding protein is a required coactivator for Smad-dependent, transforming growth factor beta transcriptional responses in endothelial cells. Proc Natl Acad Sci USA 95: 9506–9511, 1998
Wotton D, Lo RS, Lee S, Massague J: A Smad transcriptional corepressor Cell 97: 29–39, 1999
Akiyoshi S, Inoue H, Hanai J, Kusanagi K, Nemoto N, Miyazono K, Kawabata M: c-Ski acts as a transcriptional co-repressor in transforming growth factor-beta signaling through interaction with smads. J Biol Chem 274: 35269–35277, 1999
Luo K, Stroschein SL, Wang W, Chen D, Martens E, Zhou S, Zhou Q: The Ski oncoprotein interacts with the Smad proteins to repress TGFbeta signaling. Genes Dev 13: 2196–2206, 1999
Sun Y, Liu X, Eaton EN, Lane WS, Lodish HF, Weinberg RA: Interaction of the Ski oncoprotein with Smad3 regulates TGF-beta signaling. Mol Cell 4: 499–509, 1999
Stroschein SL, Wang W, Zhou S, Zhou Q, Luo K: Negative feedback regulation of TGF-beta signaling by the SnoN oncoprotein. Science 286: 771–774, 1999
Vogel P, Magert HJ, Cieslak A, Adermann K, Forssmann WG: hDIP—A potential transcriptional regulator related to murine TSC-22 and Drosophila shortsighted (shs)—is expressed in a large number of human tissues. Biochim Biophys Acta 1309: 200–204, 1996
Treisman JE, Lai ZC, Rubin GM: Shortsighted acts in the decapentaplegic pathway in Drosophila eye development and has homology to a mouse TGF-beta-responsive gene. Development 121: 2835–2845, 1995
de Caestecker MP, Yahata T, Wang D, Parks WT, Huang S, Hill CS, Shioda T, Roberts AB, Lechleider RJ: The Smad4 activation domain (SAD) is a proline-rich, p300-dependent transcriptional activation domain. J Biol Chem 275: 2115–2122, 2000
Shin HM, Seoh JY, Chung HY, Choi SJ, Hahn MJ, Kang JS, Choi MS, Han TH: Requirement of MEF2D in the induced differentiation of HL60 promyeloid cells. Mol Immunol 36: 1209–1214, 1999
Taketani S, Furukawa T, Furuyama K: Expression of coproporphyrinogen oxidase and synthesis of hemoglobin in human erythroleukemia K562 cells. Eur J Biochem 268: 1705–1711, 2001
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Choi, SJ., Moon, JH., Ahn, YW. et al. Tsc-22 enhances TGF-β signaling by associating with Smad4 and induces erythroid cell differentiation. Mol Cell Biochem 271, 23–28 (2005). https://doi.org/10.1007/s11010-005-3456-7
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11010-005-3456-7