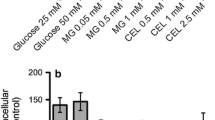
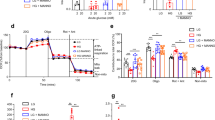
Abstract
Increased glucose flux through the hexosamine biosynthetic pathway (HBP) is known to affect the activity of a number of signal transduction pathways and lead to insulin resistance. Although widely studied in insulin responsive tissues, the effect of increased HBP activity on largely insulin unresponsive tissues, such as the brain, remains relatively unknown. Herein, we investigate the effects of increased HBP flux on Akt activation in a human astroglial cells line using glucosamine, a compound commonly used to mimic hyperglycemic conditions by increasing HBP flux. Cellular treatment with 8 mM glucosamine resulted in a 96.8% ± 24.6 increase in Akt phosphorylation after 5 h of treatment that remained elevated throughout the 9-h time course. Glucosamine treatment also resulted in modest increases in global levels of the O-GlcNAc protein modification. Increasing O-GlcNAc levels using the O-GlcNAcase inhibitor streptozotocin (STZ) also increased Akt phosphorylation by 96.8% ± 11.0 after only 3 h although for a shorter duration than glucosamine; however, the more potent O-GlcNAcase inhibitors O-(2-acetamido-2-deoxy-d-glucopyranosylidene)amino-N-phenylcarbamate (PUGNAc) and 1,2-dideoxy-2′-propyl-α-d-glucopyranoso-[2,1-d]-Δ2′-thiazoline (NAGBT) failed to mimic the increases in phospho-Akt indicating that the Akt phosphorylation is not a result of increased O-GlcNAc protein modification. Further analysis indicated that this increased phosphorylation was also not due to increased osmotic stress and was not attenuated by N-acetylcysteine eliminating the potential role of oxidative stress in the observed phospho-Akt increases. Glucosamine treatment, but not STZ treatment, did correlate with a large increase in the expression of the endoplasmic reticulum (ER) stress marker GRP 78. Altogether, these results indicate that increased HBP flux in human astroglial cells results in a rapid, short-term phosphorylation of Akt that is likely a result of increased ER stress. The mechanism by which STZ increases Akt phosphorylation, however, remains unknown.
Similar content being viewed by others
Abbreviations
- O-GlcNAc:
-
β-O-linked N-acetylglucosamine
- STZ:
-
Streptozotocin
- PUGNAc:
-
O-(2-acetamido-2-deoxy-d-glucopyranosylidene)amino-N-phenylcarbamate
- NAGBT:
-
1,2-dideoxy-2′-propyl-α-d-glucopyranoso-[2,1-d]-Δ2′-thiazoline
- ER:
-
Endoplasmic reticulum stress
References
Brownlee M (2005) The pathobiology of diabetic complications: a unifying mechanism. Diabetes 54:1615–1625
McClain DA, Lubas WA, Cooksey RC, Hazel M, Parker GJ, Love DC, Hanover JA (2002) Altered glycan-dependent signaling induces insulin resistance and hyperleptinemia. Proc Natl Acad Sci USA 99:10695–10699
Rossetti L (2000) Perspective: hexosamines and nutrient sensing. Endocrinology 141:1922–1925
Marshall S, Bacote V, Traxinger RR (1991) Discovery of a metabolic pathway mediating glucose-induced desensitization of the glucose transport system. Role of hexosamine biosynthesis in the induction of insulin resistance. J Biol Chem 266:4706–4712
Daniels MC, McClain DA, Crook ED (2000) Transcriptional regulation of transforming growth factor beta1 by glucose: investigation into the role of the hexosamine biosynthesis pathway. Am J Med Sci 319:138–142
Filippis C, Filippis A, Clark S, Proietto J (2002) Activation of PI 3-kinase by the hexosamine biosynthesis pathway. Mol Cell Endocrinol 194:29–35
Kline CL, Schrufer TL, Jefferson LS, Kimball SR (2005) Glucosamine-induced phosphorylation of the alpha-subunit of eukaryotic initiation factor 2 is mediated by the protein kinase R-like endoplasmic-reticulum associated kinase. Int J Biochem Cell Biol 38:1004–1014
Shankar RR, Zhu JS, Baron AD (1998) Glucosamine infusion in rats mimics the beta-cell dysfunction of non-insulin-dependent diabetes mellitus. Metabolism 47:573–577
Singh LP, Cook ED (2000) Hexosamine regulation of glucose-mediated laminin synthesis in mesangial cells involves protein kinases A and C. Am J Physiol Renal Physiol 279:F646–F654
Singh LP, Crook ED (2000) The effects of glucose and the hexosamine biosynthesis pathway on glycogen synthase kinase-3 and other protein kinases that regulate glycogen synthase activity. J Invest Med 48:251–258
Fujita T, Furukawa S, Morita K, Ishihara T, Shiotani M, Matsushita Y, Matsuda M, Shimomura I (2005) Glucosamine induces lipid accumulation and adipogenic change in C2C12 myoblasts. Biochem Biophys Res Commun 328:369–374
Marshall S, Nadeau O, Yamasaki K (2005) Glucosamine-induced activation of glycogen biosynthesis in isolated adipocytes. Evidence for a rapid allosteric control mechanism within the hexosamine biosynthesis pathway. J Biol Chem 280:11018–11024
Saltiel AR, Kahn CR (2001) Insulin signalling and the regulation of glucose and lipid metabolism. Nature 414:799–806
White MF (1998) The IRS-signalling system: a network of docking proteins that mediate insulin action. Mol Cell Biochem 182:3–11
Backer JM, Myers MG Jr, Shoelson SE, Chin DJ, Sun XJ, Miralpeix M, Hu P, Margolis B, Skolnik EY, Schlessinger J et al (1992) Phosphatidylinositol 3′-kinase is activated by association with IRS-1 during insulin stimulation. EMBO J␣11:3469–3479
Myers MG Jr, Backer JM, Sun XJ, Shoelson S, Hu P, Schlessinger J, Yoakim M, Schaffhausen B, White MF (1992) IRS-1 activates phosphatidylinositol 3′-kinase by associating with src homology 2 domains of p85. Proc Natl Acad Sci USA 89:10350–10354
Andjelkovic M, Alessi DR, Meier R, Fernandez A, Lamb NJ, Frech M, Cron P, Cohen P, Lucocq JM, Hemmings BA (1997) Role of translocation in the activation and function of protein kinase B. J Biol Chem 272:31515–31524
Alessi DR, James SR, Downes CP, Holmes AB, Gaffney PR, Reese CB, Cohen P (1997) Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Balpha. Curr Biol 7:261–269
Alessi DR, Andjelkovic M, Caudwell B, Cron P, Morrice N, Cohen P, Hemmings BA (1996) Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J 15:6541–6551
Sarbassov DD, Guertin DA, Ali SM, Sabatini DM (2005) Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 307:1098–1101
Andreozzi F, D’Alessandris C, Federici M, Laratta E, Del Guerra S, Del Prato S, Marchetti P, Lauro R, Perticone F, Sesti G (2004) Activation of the hexosamine pathway leads to phosphorylation of insulin receptor substrate-1 on Ser307 and Ser612 and impairs the phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin insulin biosynthetic pathway in RIN pancreatic beta-cells. Endocrinology 145:2845–2857
Nakamura M, Barber AJ, Antonetti DA, LaNoue KF, Robinson KA, Buse MG, Gardner TW (2001) Excessive hexosamines block the neuroprotective effect of insulin and induce apoptosis in retinal neurons. J Biol Chem 276:43748–43755
Vosseller K, Wells L, Lane MD, Hart GW (2002) Elevated nucleocytoplasmic glycosylation by O-GlcNAc results in insulin resistance associated with defects in Akt activation in 3T3-L1 adipocytes. Proc Natl Acad Sci USA 99:5313–5318
Manschot SM, Brands AM, van der Grond J, Kessels RP, Algra A, Kappelle LJ, Biessels GJ (2006) Brain magnetic resonance imaging correlates of impaired cognition in patients with type 2 diabetes. Diabetes 55:1106–1113
Gispen WH, Biessels GJ (2000) Cognition and synaptic plasticity in diabetes mellitus. Trends Neurosci 23:542–549
Parkes M, White KG (2000) Glucose attenuation of memory impairments. Behav Neurosci 114:307–319
Clodfelder-Miller B, De Sarno P, Zmijewska AA, Song L, Jope RS (2005) Physiological and pathological changes in glucose regulate brain Akt and glycogen synthase kinase-3. J␣Biol Chem 280:39723–39731
Corson TW, Woo KK, Li PP, Warsh JJ (2004) Cell-type specific regulation of calreticulin and Bcl-2 expression by mood stabilizer drugs. Eur Neuropsychopharmacol 14:143–150
Major EO, Miller AE, Mourrain P, Traub RG, de Widt E, Sever J (1985) Establishment of a line of human fetal glial cells that supports JC virus multiplication. Proc Natl Acad Sci USA 82:1257–1261
Hansson E (1984) Cellular composition of a cerebral hemisphere primary culture. Neurochem Res 9:153–172
Chow J, Ogunshola O, Fan SY, Li Y, Ment LR, Madri JA (2001) Astrocyte-derived VEGF mediates survival and tube stabilization of hypoxic brain microvascular endothelial cells in vitro. Brain Res Dev Brain Res 130:123–132
Barres BA (1991) Glial ion channels. Curr Opin Neurobiol 1:354–359
Goldstein GW (1988) Endothelial cell–astrocyte interactions. A cellular model of the blood-brain barrier. Ann NY Acad Sci 529:31–39
Qutub AA, Hunt CA (2005) Glucose transport to the brain: a systems model. Brain Res Brain Res Rev 49:595–617
Matthews JA, Acevedo-Duncan M, Potter RL (2005) Selective decrease of membrane-associated PKC-alpha and PKC-epsilon in response to elevated intracellular O-GlcNAc levels in transformed human glial cells. Biochim Biophys Acta 1743:305–315
Park SY, Ryu J, Lee W (2005) O-GlcNAc modification on IRS-1 and Akt2 by PUGNAc inhibits their phosphorylation and induces insulin resistance in rat primary adipocytes. Exp Mol Med 37:220–229
Gao Y, Parker GJ, Hart GW (2000) Streptozotocin-induced beta-cell death is independent of its inhibition of O-GlcNAcase in pancreatic Min6 cells. Arch Biochem Biophys 383:296–302
Haltiwanger RS, Grove K, Philipsberg GA (1998) Modulation of O-linked N-acetylglucosamine levels on nuclear and cytoplasmic proteins in vivo using the peptide O-GlcNAc-beta-N- acetylglucosaminidase inhibitor O-(2-acetamido-2-deoxy-d-glucopyranosylidene)amino-N-phenylcarbamate. J Biol Chem 273:3611–3617
Konrad RJ, Mikolaenko I, Tolar JF, Liu K, Kudlow JE (2001) The potential mechanism of the diabetogenic action of streptozotocin: inhibition of pancreatic beta-cell O-GlcNAc-selective N-acetyl-beta-d-glucosaminidase. Biochem J 356:31–41
Macauley MS, Whitworth GE, Debowski AW, Chin D, Vocadlo DJ (2005) O-GlcNAcase uses substrate-assisted catalysis: kinetic analysis and development of highly selective mechanism-inspired inhibitors. J Biol Chem 280:25313–25322
Majumdar G, Harmon A, Candelaria R, Martinez-Hernandez A, Raghow R, Solomon SS (2003) O-glycosylation of Sp1 and transcriptional regulation of the calmodulin gene by insulin and glucagon. Am J Physiol Endocrinol Metab 285:E584–591
Galvez AS, Ulloa JA, Chiong M, Criollo A, Eisner V, Barros LF, Lavandero S (2003) Aldose reductase induced by hyperosmotic stress mediates cardiomyocyte apoptosis: differential effects of sorbitol and mannitol. J Biol Chem 278:38484–38494
Pastukh V, Ricci C, Solodushko V, Mozaffari M, Schaffer SW (2005) Contribution of the PI 3-kinase/Akt survival pathway toward osmotic preconditioning. Mol Cell Biochem 269:59–67
Terada Y, Inoshita S, Hanada S, Shimamura H, Kuwahara M, Ogawa W, Kasuga M, Sasaki S, Marumo F (2001) Hyperosmolality activates Akt and regulates apoptosis in renal tubular cells. Kidney Int 60:553–567
Shaw M, Cohen P, Alessi DR (1998) The activation of protein kinase B by H2O2 or heat shock is mediated by phosphoinositide 3-kinase and not by mitogen-activated protein kinase-activated protein kinase-2. Biochem J 336(Pt 1):241–246
Tapodi A, Debreceni B, Hanto K, Bognar Z, Wittmann I, Gallyas F Jr, Varbiro G, Sumegi B (2005) Pivotal role of Akt activation in mitochondrial protection and cell survival by poly(ADP-ribose)polymerase-1 inhibition in oxidative stress. J Biol Chem 280:35767–35775
Kaneto H, Xu G, Song KH, Suzuma K, Bonner-Weir S, Sharma A, Weir GC (2001) Activation of the hexosamine pathway leads to deterioration of pancreatic beta-cell function through the induction of oxidative stress. J Biol Chem 276:31099–31104
Mastrocola R, Restivo F, Vercellinatto I, Danni O, Brignardello E, Aragno M, Boccuzzi G (2005) Oxidative and nitrosative stress in brain mitochondria of diabetic rats. J␣Endocrinol 187:37–44
Wohaieb SA, Godin DV (1987) Alterations in free radical tissue-defense mechanisms in streptozocin-induced diabetes in rat. Effects of insulin treatment. Diabetes 36:1014–1018
Fiordaliso F, Bianchi R, Staszewsky L, Cuccovillo I, Doni M, Laragione T, Salio M, Savino C, Melucci S, Santangelo F, Scanziani E, Masson S, Ghezzi P, Latini R (2004) Antioxidant treatment attenuates hyperglycemia-induced cardiomyocyte death in rats. J Mol Cell Cardiol 37:959–968
Hu P, Han Z, Couvillon AD, Exton JH (2004) Critical role of endogenous Akt/IAPs and MEK1/ERK pathways in counteracting endoplasmic reticulum stress-induced cell death. J Biol Chem 279:49420–49429
Lin HY, Masso-Welch P, Di YP, Cai JW, Shen JW, Subjeck JR (1993) The 170-kDa glucose-regulated stress protein is an endoplasmic reticulum protein that binds immunoglobulin. Mol Biol Cell 4:1109–1119
Filippis A, Clark S, Proietto J (1997) Increased flux through the hexosamine biosynthesis pathway inhibits glucose transport acutely by activation of protein kinase C. Biochem J␣324(Pt 3):981–985
Nelson BA, Robinson KA, Buse MG (2002) Defective Akt activation is associated with glucose—but not glucosamine-induced insulin resistance. Am J Physiol Endocrinol Metab 282:E497–506
Singh LP, Gennerette D, Simmons S, Crook ED (2001) Glucose-induced insulin resistance of phosphatidylinositol 3′-OH kinase and AKT/PKB is mediated by the hexosamine biosynthesis pathway. J Diabetes Complications 15:88–96
Datta K, Bellacosa A, Chan TO, Tsichlis PN (1996) Akt is a direct target of the phosphatidylinositol 3-kinase. Activation by growth factors, v-src and v-Ha-ras, in Sf9 and mammalian cells. J Biol Chem 271:30835–30839
Federici M, Menghini R, Mauriello A, Hribal ML, Ferrelli F, Lauro D, Sbraccia P, Spagnoli LG, Sesti G, Lauro R (2002) Insulin-dependent activation of endothelial nitric oxide synthase is impaired by O-linked glycosylation modification of signaling proteins in human coronary endothelial cells. Circulation 106:466–477
Miki T, Sakaue M, Kasuga M (2002) In vivo administration of glucosamine inhibited phosphatidylinositol 3-kinase activity without affecting tyrosine phosphorylation of the insulin receptor or insulin receptor substrate in rat adipocytes. Kobe J Med Sci 48:105–114
Marshall S, Nadeau O, Yamasaki K (2004) Dynamic actions of glucose and glucosamine on hexosamine biosynthesis in isolated adipocytes: differential effects on glucosamine 6-phosphate, UDP-N-acetylglucosamine, and ATP levels. J␣Biol Chem 279:35313–35319
Dong DL, Hart GW (1994) Purification and characterization of an O-GlcNAc selective N-acetyl-beta-d-glucosaminidase from rat spleen cytosol. J Biol Chem 269:19321–19330
Akimoto Y, Hart GW, Hirano H, Kawakami H (2005) O-GlcNAc modification of nucleocytoplasmic proteins and diabetes. Med Mol Morphol 38:84–91
Wells L, Vosseller K, Hart GW (2003) A role for N-acetylglucosamine as a nutrient sensor and mediator of insulin resistance. Cell Mol Life Sci 60:222–228
Musicki B, Kramer MF, Becker RE, Burnett AL (2005) Inactivation of phosphorylated endothelial nitric oxide synthase (Ser-1177) by O-GlcNAc in diabetes-associated erectile dysfunction. Proc Natl Acad Sci USA 102:11870–11875
Horal M, Zhang Z, Stanton R, Virkamaki A, Loeken MR (2004) Activation of the hexosamine pathway causes oxidative stress and abnormal embryo gene expression: involvement in diabetic teratogenesis. Birth Defects Res A Clin Mol Teratol 70:519–527
Xia Z, Nagareddy PR, Guo Z, Zhang W, McNeill JH (2006) Antioxidant N-acetylcysteine restores systemic nitric oxide availability and corrects depressions in arterial blood pressure and heart rate in diabetic rats. Free Radic Res 40:175–184
Kaufman RJ (1999) Stress signaling from the lumen of the endoplasmic reticulum: coordination of gene transcriptional and translational controls. Genes Dev 13:1211–1233
Nakatani Y, Kaneto H, Kawamori D, Yoshiuchi K, Hatazaki M, Matsuoka TA, Ozawa K, Ogawa S, Hori M, Yamasaki Y, Matsuhisa M (2005) Involvement of endoplasmic reticulum stress in insulin resistance and diabetes. J Biol Chem 280:847–851
Werstuck GH, Khan MI, Femia G, Kim AJ, Tedesco V, Trigatti B, Shi Y (2006) Glucosamine-induced endoplasmic reticulum dysfunction is associated with accelerated atherosclerosis in a hyperglycemic mouse model. Diabetes 55:93–101
Kim AJ, Shi Y, Austin RC, Werstuck GH (2005) Valproate protects cells from ER stress-induced lipid accumulation and apoptosis by inhibiting glycogen synthase kinase-3. J Cell Sci 118:89–99
Qiu W, Kohen-Avramoglu R, Mhapsekar S, Tsai J, Austin RC, Adeli K (2005) Glucosamine-induced endoplasmic reticulum stress promotes ApoB100 degradation: evidence for Grp78-mediated targeting to proteasomal degradation. Arterioscler Thromb Vasc Biol 25:571–577
Hresko RC, Heimberg H, Chi MM, Mueckler M (1998) Glucosamine-induced insulin resistance in 3T3-L1 adipocytes is caused by depletion of intracellular ATP. J Biol Chem 273:20658–20668
Kumar Y, Tatu U (2000) Induced hsp70 is in small, cytoplasmic complexes in a cell culture model of renal ischemia: a comparative study with heat shock. Cell Stress Chaperones 5:314–327
Lenk SE, Bhat D, Blakeney W, Dunn WA Jr (1992) Effects of streptozotocin-induced diabetes on rough endoplasmic reticulum and lysosomes of rat liver. Am J Physiol 263:E856–862
Kruglikov I, Gryshchenko O, Shutov L, Kostyuk E, Kostyuk P, Voitenko N (2004) Diabetes-induced abnormalities in ER calcium mobilization in primary and secondary nociceptive neurons. Pflugers Arch 448:395–401
Kawakami Y, Nishimoto H, Kitaura J, Maeda-Yamamoto M, Kato RM, Littman DR, Leitges M, Rawlings DJ, Kawakami T (2004) Protein kinase C betaII regulates Akt phosphorylation on Ser-473 in a cell type- and stimulus-specific fashion. J Biol Chem 279:47720–47725
Perez-Garcia MJ, Cena V, de Pablo Y, Llovera M, Comella JX, Soler RM (2004) Glial cell line-derived neurotrophic factor increases intracellular calcium concentration. Role of calcium/calmodulin in the activation of the phosphatidylinositol 3-kinase pathway. J Biol Chem 279:6132–6142
Dudek H, Datta SR, Franke TF, Birnbaum MJ, Yao R, Cooper GM, Segal RA, Kaplan DR, Greenberg ME (1997) Regulation of neuronal survival by the serine-threonine protein kinase Akt. Science 275:661–665
Kohout SC, Corbalan-Garcia S, Torrecillas A, Gomez-Fernandez JC, Falke JJ (2002) C2 domains of protein kinase C isoforms alpha, beta, and gamma: activation parameters and calcium stoichiometries of the membrane-bound state. Biochemistry 41:11411–11424
Acknowledgements
We would like to thank Dr. David J. Vocadlo at Simon Fraser University in British Columbia, Canada (Department of Chemistry) for the kind gift of NAGBT and helpful discussions and Dr. Gerald Hart at Johns Hopkins University School of Medicine (Department of Biological Chemistry) for the gift of CTD 110.6 antibody.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Matthews, J.A., Belof, J.L., Acevedo-Duncan, M. et al. Glucosamine-induced increase in Akt phosphorylation corresponds to increased endoplasmic reticulum stress in astroglial cells. Mol Cell Biochem 298, 109–123 (2007). https://doi.org/10.1007/s11010-006-9358-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-006-9358-5