Abstract
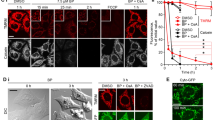
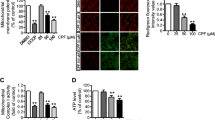
To elucidate the significance of mitochondrial localization of Cu/Zn-SOD (SOD1), we studied the relationship between the release of mitochondrial SOD1 and apoptosis. Kinetic analysis using HL-60 cells showed that both mitochondria-dependent and mitochondria-independent pro-apoptotic drugs, such as staurosporine and actinomycin D, increased the generation of reactive oxygen species (ROS) and decreased mitochondrial membrane potential (Δψ). ROS generation by these drugs was inhibited by Mn (III) tetrakis (5,10,15,20-benzoic acid) porphyrin (MnTBAP), a cell membrane-permeable SOD mimetic. However, MnTBAP inhibited the apoptosis induced by staurosporine but not by actinomycin D. MnTBAP failed to inhibit Δψ decrease and release of SOD1 and cytochrome c induced by actinomycin D. Moreover, 4,4′-diisothiocyanatostilbene-2,2′-disulfonic acid (DIDS), an inhibitor of voltage-dependent anion channel (VDAC), inhibited the release of the two proteins and apoptosis induced by staurosporine but not actinomycin D. These results suggest that ROS plays an important role in mitochondria-dependent but not mitochondria-independent apoptosis and that the release of SOD1 increases the susceptibility of mitochondria to oxidative stress, thereby enhancing a vicious cycle leading to apoptosis.






Similar content being viewed by others
References
Kerr JF, Wyllie AH, Currie AR (1972) Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer 26:239–257
Wyllie AH (1980) Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature 284:555–556
Crompton M (1999) The mitochondrial permeability transition pore and its role in cell death. Biochem J 341(Pt 2):233–249
Green DR, Reed JC (1998) Mitochondria and apoptosis. Science 281:1309–1312
Gogvadze V, Robertson JD, Zhivotovsky B et al (2001) Cytochrome c release occurs via Ca2+ -dependent and Ca2+ -independent mechanisms that are regulated by Bax. J Biol Chem 276:19066–19071
Liu X, Kim CN, Yang J et al (1996) Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell 86:147–157
Zou H, Henzel WJ, Liu X et al (1997) Apaf-1, a human protein homologous to C. elegans CED-4, participates in cytochrome c-dependent activation of caspase-3. Cell 90:405–413
Kira Y, Sato EF, Inoue M (2002) Association of Cu, Zn-type superoxide dismutase with mitochondria and peroxisomes. Arch Biochem Biophys 399:96–102
Okado-Matsumoto A, Fridovich I (2001) Subcellular distribution of superoxide dismutases (SOD) in rat liver: Cu, Zn-SOD in mitochondria. J Biol Chem 276:38388–38393
Simon HU, Haj-Yehia A, Levi-Schaffer F (2000) Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis 5:415–418
Vincent AM, Brownlee M, Russell JW (2002) Oxidative stress and programmed cell death in diabetic neuropathy. Ann N Y Acad Sci 959:368–383
Kanno T, Sato EE, Muranaka S et al (2004) Oxidative stress underlies the mechanism for Ca(2+)-induced permeability transition of mitochondria. Free Radic Res 38:27–35
Cai J, Jones DP (1998) Superoxide in apoptosis. Mitochondrial generation triggered by cytochrome c loss. J Biol Chem 273:11401–11404
Boveris A, Oshino N, Chance B (1972) The cellular production of hydrogen peroxide. Biochem J 128:617–630
Muller FL, Liu Y, Van Remmen H (2004) Complex III releases superoxide to both sides of the inner mitochondrial membrane. J Biol Chem 279:49064–49073
Himmelfarb J, Lazarus JM, Hakim R (1991) Reactive oxygen species production by monocytes and polymorphonuclear leukocytes during dialysis. Am J Kidney Dis 17:271–276
Facompre M, Wattez N, Kluza J et al (2000) Relationship between cell cycle changes and variations of the mitochondrial membrane potential induced by etoposide. Mol Cell Biol Res Commun 4:37–42
Levine RL, Williams JA, Stadtman ER et al (1994) Carbonyl assays for determination of oxidatively modified proteins. Methods Enzymol 233:346–357
Liu S, Kawai K, Tyurin VA et al (2001) Nitric oxide-dependent pro-oxidant and pro-apoptotic effect of metallothioneins in HL-60 cells challenged with cupric nitrilotriacetate. Biochem J 354:397–406
Flitter WD, Mason RP (1988) The enzymatic reduction of actinomycin D to a free radical species. Arch Biochem Biophys 267:632–639
Gil J, Almeida S, Oliveira CR et al (2003) Cytosolic and mitochondrial ROS in staurosporine-induced retinal cell apoptosis. Free Radic Biol Med 35:1500–1514
Li Q, Sato EF, Kira Y et al (2006) A possible cooperation of SOD1 and cytochrome c in mitochondria-dependent apoptosis. Free Radic Biol Med 40:173–181
Koopman G, Reutelingsperger CP, Kuijten GA et al (1994) Annexin V for flow cytometric detection of phosphatidylserine expression on B cells undergoing apoptosis. Blood 84:1415–1420
Vermes I, Haanen C, Steffens-Nakken H et al (1995) A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J Immunol Methods 184:39–51
Anderson KM, Alrefai W, Bonomi P et al (2003) Caspase-dependent and -independent panc-1 cell death due to actinomycin D and MK 886 are additive but increase clonogenic survival. Exp Biol Med (Maywood) 228:915–925
Anderson KM, Bonomi P, Hu Y et al (2003) An interaction between type 1 and type 2 programmed cell death and clonogenic survival. Med Hypotheses 61:583–585
Yuste VJ, Sanchez-Lopez I, Sole C et al (2002) The prevention of the staurosporine-induced apoptosis by Bcl-X(L), but not by Bcl-2 or caspase inhibitors, allows the extensive differentiation of human neuroblastoma cells. J Neurochem 80:126–139
Kruman I, Guo Q, Mattson MP (1998) Calcium and reactive oxygen species mediate staurosporine-induced mitochondrial dysfunction and apoptosis in PC12 cells. J Neurosci Res 51:293–308
Ikeda K, Kajiwara K, Tanabe E et al (1999) Involvement of hydrogen peroxide and hydroxyl radical in chemically induced apoptosis of HL-60 cells. Biochem Pharmacol 57:1361–1365
Moore RM, Lundgren DW, Moore JJ (1999) Cyclooxygenase inhibitors decrease apoptosis initiated by actinomycin D, cycloheximide, and staurosporine in amnion-derived WISH cells. J Soc Gynecol Investig 6:245–251
Matsura T, Serinkan BF, Jiang J et al (2002) Phosphatidylserine peroxidation/externalization during staurosporine-induced apoptosis in HL-60 cells. FEBS Lett 524:25–30
Pong K, Doctrow SR, Huffman K et al (2001) Attenuation of staurosporine-induced apoptosis, oxidative stress, and mitochondrial dysfunction by synthetic superoxide dismutase and catalase mimetics, in cultured cortical neurons. Exp Neurol 171:84–97
Shim D, Kang HY, Jeon BW et al (2004) Protein kinase B inhibits apoptosis induced by actinomycin D in ECV304 cells through phosphorylation of caspase 8. Arch Biochem Biophys 425:214–220
Debatin KM, Poncet D, Kroemer G (2002) Chemotherapy: targeting the mitochondrial cell death pathway. Oncogene 21:8786–8803
Bruijn LI, Becher MW, Lee MK et al (1997) ALS-linked SOD1 mutant G85R mediates damage to astrocytes and promotes rapidly progressive disease with SOD1-containing inclusions. Neuron 18:327–338
Higgins CM, Jung C, Xu Z (2003) ALS-associated mutant SOD1G93A causes mitochondrial vacuolation by expansion of the intermembrane space and by involvement of SOD1 aggregation and peroxisomes. BMC Neurosci 4:16
Liu R, Li B, Flanagan SW et al (2002) Increased mitochondrial antioxidative activity or decreased oxygen free radical propagation prevent mutant SOD1-mediated motor neuron cell death and increase amyotrophic lateral sclerosis-like transgenic mouse survival. J Neurochem 80:488–500
Shibata N (2001) Transgenic mouse model for familial amyotrophic lateral sclerosis with superoxide dismutase-1 mutation. Neuropathology 21:82–92
Chiu AY, Zhai P, Dal Canto MC et al (1995) Age-dependent penetrance of disease in a transgenic mouse model of familial amyotrophic lateral sclerosis. Mol Cell Neurosci 6:349–362
Rouleau GA, Clark AW, Rooke K et al (1996) SOD1 mutation is associated with accumulation of neurofilaments in amyotrophic lateral sclerosis. Ann Neurol 39:128–131
Mattiazzi M, D’Aurelio M, Gajewski CD et al (2002) Mutated human SOD1 causes dysfunction of oxidative phosphorylation in mitochondria of transgenic mice. J Biol Chem 277:29626–29633
Guegan C, Vila M, Rosoklija G et al (2001) Recruitment of the mitochondrial-dependent apoptotic pathway in amyotrophic lateral sclerosis. J Neurosci 21:6569–6576
Acknowledgments
This work was supported by Special Coordination Funds for Promoting Science and Technology from the Ministry of Education, Culture, Sports, Science and Technology (16590252 and 14370062), and 21st Century COE Program “Base to Overcome Fatigue” supported by MEXT, Japan. This work was also supported by the Sasakawa Scientific Research Grant from The Japan Science Society. The authors thank Prof. S. Tsuyoshi Ohnishi for his critical reading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, Q., Sato, E.F., Zhu, X. et al. A simultaneous release of SOD1 with cytochrome c regulates mitochondria-dependent apoptosis. Mol Cell Biochem 322, 151–159 (2009). https://doi.org/10.1007/s11010-008-9952-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-008-9952-9