Abstract
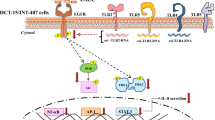
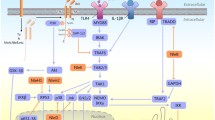
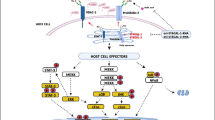
Enteroaggregative Escherichia coli (EAEC) is emerging as a cause of acute and persistent diarrhea in developing countries. An important feature of EAEC pathogenesis is the induction of profound inflammatory response in the intestinal epithelium. In this article, we have shown that EAEC-induced activation of mitogen-activated protein kinases (MAPK) (ERK-1/2, JNK and p38MAPK) in cultured human intestinal epithelial cells (INT-407) leads to the induction of DNA-binding activity of NF-κB and AP-1, resulting in IL-8 production. Plasmid-cured EAEC could also activate the MAPK and the transcription factors leading to IL-8 secretion, but to a lesser extent than that of wild-type EAEC. Further, pretreatment of these cells with the highly specific MEK inhibitor (PD 098059), the JNK inhibitor (SP 600125), and the p38MAPK inhibitor (SB 203580) resulted in inhibition of the IL-8 secretion by EAEC (wild type as well as plasmid cured)-infected INT-407 cells. These findings demonstrate that the inflammatory response induced by EAEC may be due to the specific stimulation of MAPK signaling pathways leading to nuclear responses. To our knowledge, this is the first article regarding the detailed mechanism of IL-8 secretion from the EAEC-infected human intestinal epithelial cell line.





Similar content being viewed by others
References
Huang DB, DuPont HL (2004) Enteroaggregative Escherichia coli: an emerging pathogen in children. Semin Pediatr Infect Dis 15:266–271
Pawlowski SW, Warren CA, Guerrant R (2009) Diagnosis and treatment of acute or persistent diarrhea. Gastroenterology 136:1874–1886
Okeke IN, Nataro JP (2001) Enteroaggregative Escherichia coli. Lancet Infect Dis 1:304–313
Steiner TS, Lima AA, Nataro JP, Guerrant RL (1998) Enteroaggregative Escherichia coli produce intestinal inflammation and growth impairment and cause interleukin-8 release from intestinal epithelial cells. J Infect Dis 177:88–96
Steiner TS, Nataro JP, Poteet-Smith CE, Smith JA, Guerrant RL (2000) Enteroaggregative Escherichia coli expresses a novel flagellin that causes IL-8 release from intestinal epithelial cells. J Clin Invest 105:1769–1777
Harrington SM, Strauman MC, Abe CM, Nataro JP (2005) Aggregative adherence fimbriae contribute to the inflammatory response of epithelial cells infected with enteroaggregative Escherichia coli. Cell Microbiol 7:15651–15678
Goyal A, Bhattacharyya S, Majumdar S, Narang A, Ghosh S (2009) Cellular response induced by a galactose-specific adhesin of enteroaggregative Escherichia coli in INT-407 cells. FEMS Immunol Med Microbiol 55:378–387
Mukaida N, Okamoto S, Ishikawa Y, Matsushima K (1994) Molecular mechanism of interleukin-8 gene expression. J Leukoc Biol 56:554–558
Dahan S, Busuttil V, Imbert V, Peyron JF, Rampal P, Czerucka D (2002) Enterohemorrhagic Escherichia coli infection induces interleukin-8 production via activation of mitogen-activated protein kinases and the transcription factors NF-kappaB and AP-1 in T84 cells. Infect Immun 70:2304–2310
Hobbie S, Chen LM, Davis RJ, Galan JE (1997) Involvement of mitogen-activated protein kinase pathways in the nuclear responses and cytokine production induced by Salmonella typhimurium in cultured intestinal epithelial cells. J Immunol 9:5550–5559
Savkovic SD, Ramaswamy A, Koutsouris A, Hecht G (2001) EPEC-activated ERK1/2 participate in inflammatory response but not tight junction barrier disruption. Am J Physiol Gastrointest Liver Physiol 281:6890–6898
Khan MA, Kang J, Steiner TS (2004) Enteroaggregative Escherichia coli flagellin-induced interleukin-8 secretion requires Toll-like receptor 5-dependent p38 MAP kinase activation. Immunology 112:651–660
Mesas JM, Carmen RM, Alegre MT (2004) Plasmid curing of Oenococcus oeni. Plasmid 51:37–40
Schmidt H, Knop C, Franke S, Aleksic S, Heesemann J, Karch H (1995) Development of PCR for screening of enteroaggregative Escherichia coli. J Clin Microbiol 33:701–705
Cravioto A, Tello A, Navarro A, Ruiz J, Villafan H, Uribe F, Eslava C (1991) Association of Escherichia coli HEp-2 adherence patterns with type and duration of diarrhoea. Lancet 337:262–264
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Towbin H, Staehelin T, Gordon J (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA 76:4350–4354
Cerna JF, Nataro JP, Estrada-Garcia T (2003) Multiplex PCR for detection of three plasmid-borne genes of enteroaggregative Escherichia coli strains. J Clin Microbiol 41:2138–2140
Nataro JP, Hicks S, Phillips AD, Vial PA, Sears CL (1996) T84 cells in culture as a model for enteroaggregative Escherichia coli pathogenesis. Infect Immun 64:4761–4768
Hicks S, Candy DC, Phillips AD (1996) Adhesion of enteroaggregative Escherichia coli to pediatric intestinal mucosa in vitro. Infect Immun 64:4751–4760
Lerman LS (1963) The structure of the DNA-acridine complex. Proc Natl Acad Sci USA 49:94–102
Waring MJ (1968) Drugs which affects the structure and function of DNA. Nature 219:1320–1325
Crick FHC, Barnett L, Brenner S, Watts-Tobin R (1961) General nature of the genetic code for proteins. Nature 192:1227–1232
Jiang ZD, Greenberg D, Nataro JP, Steffen R, DuPont HL (2002) Rate of occurrence and pathogenic effect of enteroaggregative Escherichia coli virulence factors in international travelers. J Clin Microbiol 40:4185–4190
Huang DB, Mohanty A, DuPont HL, Okhuysen PC, Chiang T (2006) A review of an emerging enteric pathogen: enteroaggregative Escherichia coli. J Med Microbiol 55:1303–1311
Meyer CF, Wang X, Chang C, Templeton D, Tan TH (1996) Interaction between c-Rel and the mitogen-activated protein kinase kinase kinase 1 signaling cascade in mediating kappaB enhancer activation. J Biol Chem 271:8971–8976
Karin M (1995) The regulation of AP-1 activity by mitogen-activated protein kinases. J Biol Chem 270:16483–16486
Hobert ME, Sands KA, Mrsny RJ, Madara JL (2002) Cdc42 and Rac1 regulate late events in Salmonella typhimurium induced interleukin-8 secretion from polarized epithelial cells. J Biol Chem 277:51025–51032
Kim SY, Lee YC, Kim HK, Blaser MJ (2006) Helicobacter pylori CagA transfection of gastric epithelial cells induces interleukin-8. Cell Microbiol 8:97–106
Betis F, Brest P, Hofman V, Guignot J, Bernet-Camard MF, Rossi B, Servin A, Hofman P (2003) The Afa/Dr adhesins of diffusely adhering Escherichia coli stimulate interleukin-8 secretion, activate mitogen-activated protein kinases, and promote polymorphonuclear transepithelial migration in T84 polarized epithelial cells. Infect Immun 71:1068–1074
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khan, K., Konar, M., Goyal, A. et al. Enteroaggregative Escherichia coli infection induces IL-8 production via activation of mitogen-activated protein kinases and the transcription factors NF-κB and AP-1 in INT-407 cells. Mol Cell Biochem 337, 17–24 (2010). https://doi.org/10.1007/s11010-009-0282-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-009-0282-3