Abstract
Dermatophytosis is a relatively common disease in many countries occurring endemically both in companion and food animals. Fungi belonging to the genera Trichophyton and Microsporum are most often isolated from clinical cases. Measures to control and prevent dermatophytosis include sanitation, hygienic measures and treatment. In some countries, successful control and eradication have been achieved by mass vaccination of cattle and fur-bearing animals. Vaccines containing live attenuated cells of the fungus stimulate a cell-mediated immune response conferring long-lasting protection against subsequent challenge by the homologous fungus. A delayed type hypersensitivity (DTH) skin test using appropriate dermatophyte antigens is suitable to assess the response. Inactivated dermatophyte vaccines are available for use in cattle, horse, dog, and cat in some countries. However, the scientific literature is scarce making it difficult to conclude on efficacy and appropriate use. Current vaccines are all first generation vaccines. Attempts have been made to prepare subunit vaccines based on new knowledge about virulence factors like the keratinases, so far with limited success. Candidate antigens must be able to stimulate a strong T helper 1 cell response and future research should focus on identification of major T-cell epitopes that specifically elicit a DTH reaction. Dermatophytosis is a zoonotic disease. In Norway and a few other countries, systematic vaccination against cattle ringworm has almost eliminated the disease, and ringworm in man caused by T. verrucosum is almost nonexistent. A similar benefit could be expected if a safe and efficacious vaccine was available for Microsporum canis infection in cats and dogs.
Similar content being viewed by others
Introduction
Dermatophytosis or ringworm is a fungal infection of the hair and of the superficial keratinized cell layers of the skin occurring in animals and man. Several species of genus Microsporum or genus Trichophyton belonging to the groups of zoophilic or geophilic dermatophytes can cause clinical infections in animals. There is a certain degree of host specificity, but some of the dermatophyte species may infect a variety of hosts, including man [1, 2]. Upon introduction to a herd, kennel or cattery, the disease spreads easily from one animal to another by direct transmission or indirectly via environmental contamination or fomites. Measures to prevent and control dermatophytosis have included sanitation, hygienic measures, prophylactic treatment, and vaccines have been available against ringworm in some animal species [3, 4]. In Norway and a few other countries, ringworm in cattle and fur-bearing animals is a notifiable disease. To limit spread, bio-security measures are imposed on affected herds, e.g., restrictions on sale of animals, access to common grasslands, and participation in shows.
In the endemic situation, ringworm is a disease of the young animal. Naturally occurring ringworm is seldom recurrent suggesting an effective and long-lasting immunity, and observations from experimental studies confirm that animals express increased resistance to subsequent challenge by the homologous fungus [5–7]. Successful resolution of lesions correlates with development of a cell-mediated immune response as demonstrated by a delayed type hypersensitivity (DTH) skin reaction [8, 9]. As pointed out by Smith and Griffin [10], this knowledge must form the basis for vaccine development against ringworm.
In the 1960s, Kielstein and coworkers [11] used inactivated preparations of Trichophyton verrucosum for vaccination of cattle, but with limited success. Pioneering work was carried out by researchers at the All-Union Institute of Experimental Veterinary Science (VIEV), Moscow, in the late 1960s and 1970s, reviewed by Tretiakov et al. [12] and Sarkisov [13]. A large number of field isolates of dermatophytes from different animal species belonging to the genus Trichophyton was characterized and sub-cultured in the process of attenuation. Strains producing abundant numbers of microconidia were selected for vaccine production [13]. This procedure of attenuation was used for T. verrucosum (VIEV culture collection strain 130), T. equinum (VIEV culture collection strain 2251/70), and T. mentagrophytes (VIEV culture collection strain 1024) [14–16] and vaccine formulations were developed for cattle, horse, and farmed fox, respectively. Efficacy and safety were demonstrated in experimental studies and field trials [13]. During the 1970s, the Russian vaccine against cattle ringworm was introduced in Bulgaria [17], Hungary [18], former German Democratic Republic [19], former Yugoslavia [20], and in Norway as the first country in Western Europe [21]. In former Czechoslovakia, a T. verrucosum vaccine containing live cells of the strain CCM 8165 (A Rybnikar, personal communication) was available from 1974 and manufactured by a national company [22].
Several attempts have been made to develop a vaccine against Microsporum canis infection in dogs and cats; however, a well documented, safe and efficacious vaccine is still lacking [3].
In this article, we review immunoprophylaxis against dermatophyte infections in companion animals and in fur-bearing and food animals.
Host-Fungus Relationships of Importance for Vaccination and Protection
Host Immune Response
The immunopathogenesis of dermatophyte infections is reviewed in a previous chapter in this issue of Mycopathologia, therefore, only some aspects of the host immune response important for protection against disease will be discussed here. Both innate and adaptive immune mechanisms are involved in the response to a dermatophyte infection. Furthermore, it has been demonstrated that antigens of Microsporum canis and several species belonging to the genus Trichophyton elicit both humoral and cell-mediated immune responses [23–26]. Dermatophytosis seems to be another example of a disease illustrating the dichotomy of the immune system and outcome of the infection. Predominant activation of T helper type 2 (Th2) cells and corresponding cytokine profile leads to antibody formation and is associated with a chronic disease [10, 26, 27]. Activation of Th1 cells stimulates a cell-mediated response characterized by the cytokines interferon-γ (IFN-γ), interleukin 12 (IL-12), and IL-2 leading to recovery [10, 26, 27]. Passive transfer of anti-dermatophyte antibodies does not protect against challenge with the homologous fungus in a mouse model [28], whereas transfer of lymphoid cells from infected donors confers protection to susceptible recipients. A positive DTH reaction confirms that a cell-mediated immune response has been established. Conversely, an immediate hypersensitivity skin test reaction indicates a humoral response. IFN-γ plays an important role in the effector phase of the DTH reaction [29]. In patients with dermatophytosis, in vitro release of IFN-γ by peripheral blood mononuclear cells has been demonstrated [30], and in those with a chronic course of infection a decreased IFN-γ production was detected upon stimulation with trichophytin [31]. Experimental inoculation of calves with a virulent strain of T. verrucosum resulted in IFN-γ production in cultures of whole blood cells sampled 4 weeks post infection and stimulated by trichophytin [32]. Furthermore, vaccination of calves with a live attenuated T. verrucosum vaccine (Bovilis® Ringvac, Intervet, the Netherlands), revealed production of IFN-γ indicating stimulation of a cell-mediated immune response [32]. Vaccinated calves were protected upon challenge. In conclusion, an efficient dermatophyte vaccine must stimulate appropriate lymphocyte subsets which prime the animal for a subsequent cell-mediated immune response leading to clearance of the fungus and recovery from infection [10]. A DTH skin test using appropriate dermatophyte antigen is suitable to assess the type of immune response.
Dermatophyte Antigens
Initial studies by Sarkisov and co-workers [13] demonstrated that vaccines containing hyphal elements of dermatophytes did not confer protection upon challenge, whereas vaccines of conidia from the same dermatophytes did. Hussin and Smith [7] examined immunogenicity and protection of different vaccine preparations from T. mentagrophytes in a guinea pig model. The live spore vaccine was the most effective and the authors suggested that antigens responsible for a protective immune response are likely to be exposed at time of spore germination and early hyphal growth. The cytoplasmic extract vaccine had no beneficial effect on the course of infection, whereas the cell wall antigen vaccine prepared from inactivated hyphae cells produced an intermediate type of protection [7]. The efficacy of an M. canis cell wall vaccine was examined in kittens both by direct challenge and contact exposure with infected animals [33, 34]. However, all animals developed ringworm lesions. Calves and guinea pigs were vaccinated with preparations from the ribosomal fraction of T. verrucosum and M. canis, respectively [35, 36]. Signs of ringworm developed after challenge in both trials, but the duration of the course of infection was significantly reduced compared to mock-vaccinated animals. The antigens used in these experimental vaccines were not well characterized.
Typical for dermatophytes is the production of keratinases considered as virulence factors playing a role in the pathogenesis of infection [37–39]. There are two families of keratinolytic enzymes, the subtilase family, which are serine proteases, and the zinc metalloprotease family. In a guinea pig model, humoral and cell-mediated immune responses to a recombinant metalloprotease from M. canis have been described [40]. However, a subunit vaccine based on this enzyme [41] or a subtilase [42] failed to protect the animals against development of skin lesions. Interestingly, Woodfolk and co-workers [26, 43] demonstrated that a subtilase recombinant enzyme derived from T. rubrum, Tri r 2, had the ability to stimulate immediate and delayed-type hypersensitivity skin test reactions in different test persons. Furthermore, the peptide P5 was an immunodominant epitope of the Tri r 2 specifically associated with the DTH. In the search for protective immunogens, peptides specifically stimulating strong memory T-cell responses are candidate vaccine antigens. Raska and co-workers [44] examined the potential protective effects in calves and guinea pigs of two vaccine formulations based on heat shock proteins (hsp) from T. mentagrophytes and compared with commercial inactivated and live T. verrucosum vaccines. The animals were challenged by epicutaneous inoculation of a virulent strain of T. mentagrophytes. The DNA plasmid vaccine designated pVAX1–hsp60–TM814K seemed to confer some protection especially in the guinea pig model. The recombinant Freund adjuvanted protein vaccine designated r-hsp60–TM664 gave highly variable results. For both vaccine products, it is not possible to draw conclusions because of the small number of animals in each group and of different vaccine compositions with respect to antigen amount and adjuvant content. Therefore, current dermatophyte vaccines are first generation vaccines with inactivated or attenuated live fungal cells.
Vaccination Against Dermatophytosis in Cattle
In cattle, dermatophytosis is most often caused by T. verrucosum; occasionally T. mentagrophytes, T. equinum, and M. canis are isolated from ringworm lesions [1, 4]. There are four commercialized vaccine products against cattle ringworm caused by T. verrucosum (Table 1); three are monovalent, containing live cells of T. verrucosum [45, 46], whereas the inactivated vaccine contains the three dermatophyte species T. verrucosum, T. mentagrophytes, and T. sarkisovii (now synonymous with T. mentagrophytes on the basis of recent molecular studies) [47]. The most common procedure for evaluation of vaccine efficacy and safety and characterization of the immune response involves the target animal species; moreover, these parameters are studied in a homologous system where animals are challenged with a virulent strain belonging to the same dermatophyte species as in the vaccine. A few experiments have used heterologous challenge strains demonstrating some degree of cross reactions [48, 49].
Inactivated Dermatophyte Vaccines
Vaccines for cattle containing formalin-inactivated cells of strains of T. verrucosum have been examined for immunogenicity and protective ability in experimental and field trials [50, 51]. Vaccination of calves aged 5–8 days stimulated a cellular immune response assessed by skin test and in a leukocyte migration inhibition test. In an experimental trial, some protection was demonstrated after epicutaneous challenge of calves with a virulent strain of T. verrucosum [50]. However, in a field trial, the design included vaccination of already infected animals and was considered suboptimal for the evaluation of prophylactic efficacy of the vaccine preparation [51]. Limited information is available regarding efficacy and duration of immunity of the inactivated trivalent vaccine Insol® Trichophyton (Boehringer Ingelheim Vetmedica GmbH, Germany), but annual revaccination is recommended [47]. In a blinded challenge study, comparison was made between this vaccine and a monovalent live attenuated vaccine (Bovilis® Ringvac, Intervet, the Netherlands) [52]. None of the calves vaccinated with the live vaccine developed ringworm signs, whereas all calves vaccinated with the inactivated vaccine had severe ringworm lesions comparable to the control group (Table 2). The difference between live and inactivated T. verrucosum vaccine preparations in stimulating protective immunity has been confirmed in guinea pigs and calves [53].
Dermatophyte Vaccines Containing Live Fungal Cells
The vaccine strains T. verrucosum LTF-130 (Bovilis® Ringvac, Intervet, the Netherlands) [45] and CCM 8165 (Trichoben, Bioveta Inc., Czech Republic) [46] are both characterized by an abundant production of microconidia (Fig. 1) and remaining virulence properties. Interestingly, the Czech research group has further attenuated the CCM 8165 vaccine strain by ultraviolet light-induced mutagenesis [54] producing an avirulent strain designated TV-M-310 (Trichoben AV) [46]. Efficacy has been demonstrated for these vaccines in experimental trials with commingling exposure (i.e., a study in which the test animals are challenged by exposure to an actively infected animal mixed in with the group) and epicutaneous inoculation of a virulent strain of T. verrucosum in the target animal [55–57]. Protective immunity in animals vaccinated with the two first mentioned products has been compared and found to be equally satisfactory [56]. In vaccinated calves, epicutaneous inoculation of the virulent strain of T. verrucosum elicits a mild inflammatory reaction appearing a couple of days post challenge and resolving within 4–6 days (Table 2) [52]. This reaction is a visible evidence of the anamnestic immune response involving rapid mobilization of effector cells leading to elimination of the fungus.
Field trials have been conducted in areas with high prevalence of bovine ringworm and all have demonstrated significant reduction of cases of disease and concluded that vaccination should be part of a ringworm control program [58–60]. For the purpose of obtaining experience with the Russian ringworm vaccine under Norwegian conditions, a field trial was conducted in an area of dairy production with a herd prevalence rate of ringworm of 70% [59]. For 6 years, approximately 95% of all herds in the area participated in the program, which included vaccination of non-infected animals of all ages, followed by vaccination of all calves born and purchased animals. The prevalence rate of ringworm-infected herds was reduced to 0% 8 years after inception of the program.
The vaccination schedule consists of intramuscular injection of two doses with an interval of 10–14 days. One dose or doubling the dose given once does not confer satisfactory protection [61]. The significance of the fungal antigen amount to trigger a protective immune response has been studied [62] and was further demonstrated in a field trial in calves in specialized beef production [63]. Under these conditions, the vaccine dose had to be doubled of that recommended, to produce a statistically significant preventive effect. Newborn calves can be vaccinated [64–66] and there is no interference with maternal antibodies from previously vaccinated dams [66, 67]. Onset of immunity has been studied in calves and when challenged at different time points after the last vaccination, a gradual increase in protection was observed [56]. In calves challenged 14–25 days after the last vaccination, up to 10% developed mild signs of ringworm, and when challenged at 28 days, only one of 108 calves presented mild signs of infection at the inoculation site. All animals challenged 35 days after the last vaccination were fully protected.
Field experience indicates that duration of immunity is long lasting following vaccination with live dermatophyte vaccines and no booster vaccination is recommended [12, 68]. Rybnikar and co-workers [56] demonstrated full protection of all animals challenged 1 year after vaccination. The authors commented on Russian experiments where a few cattle were challenged by epicutaneous inoculation of a virulent strain of T. verrucosum 1 year after vaccination and others after 3 years. Signs of ringworm were observed in one of three and one of five animals, respectively. No reason for this outcome was presented, but the inoculated dose of the fungus may have been very high and in a sense overwhelmed the vaccine-induced immunity. Moreover, epicutaneous inoculation of the fungus onto depilated and sometimes slightly scarified skin is rather different from natural exposure. Vaccine failure has been reported [57, 58], and likely explanations may be occurrence of other dermatophyte species than T. verrucosum, notably M. canis and T. equinum, incorrect vaccination procedure, high level of natural challenge exposure, or vaccination during the incubation period.
The vaccine strains used in live vaccines are attenuated, but have some remaining virulence properties [69]. Following intramuscular injection, a lesion may develop, 1–2 cm in diameter with some scaling or moderate hair loss and crust formation (Fig. 2) [45, 46, 70]. Regression of the lesion is seen within 2–4 weeks. Histological examination of the skin lesion reveals presence of fungal hyphae in hair follicles accompanied by an inflammatory reaction with influx of neutrophils and CD4+ T cells (Fig. 3). The development of the lesion confirms that the vaccine strain has retained certain virulence properties likely to be crucial for the stimulation of an appropriate protective immune response. Culture of specimens from the skin surface yields the vaccine strain (69, AM Bratberg, personal communication). In commingling trials, examination of transmission of the vaccine strain from calves presenting lesions at the injection site to non-vaccinated calves, revealed no transfer (AM Bratberg, personal communication). Direct inoculation of skin scrapings from the lesion onto skin of non-vaccinated calves did not produce any skin reaction, most likely because of low number of fungal cells in the inoculum material (AM Bratberg, personal communication). Regarding safety of humans using the vaccine or being exposed by contact with vaccinated animals, the authors are not aware of any published reports of suspected disease in humans associated with the vaccines containing attenuated strains of T. verrucosum. However, the manufacturers underscore that the vaccine should not be used by immunosuppressed individuals [45] and the need for gloves when vaccinating [46].
Immunohistochemical staining of cryosection of biopsy sampled from the injection site in calf 12 days after vaccination with Bovilis® Ringvac (Intervet, the Netherlands) (×(400). T. verrucosum stained green with FITC-labeled goat anti-rabbit antibodies (primary antibody: rabbit polyclonal T. verrucosum antibodies, National Veterinary Institute, Oslo, Norway). CD4 + T-cells stained red with phycoerythrin-labeled goat anti-mouse antibodies (primary antibody: IL-A12, ILRI, Nairobi, Kenya) (Lund and Bratberg, unpublished)
A Russian-attenuated Trichophyton vaccine, Vermet, was recently described in a preliminary report as efficacious in multiple species, including cattle, goats, camels, rabbits, and foxes [71]. No additional information or studies on this product have come forth.
Vaccination in Control and Eradication Programs
In the former Soviet Union, bovine ringworm was common and considered present in all farms in the 1960s [13]. A nationwide campaign was initiated in 1969, and approximately 250 million cattle were vaccinated in the period 1970–1980 [12]. Systematic mass vaccination was promising and in 1975 less than 10% of the farms were infected [72]. The prevalence decreased further to less than 1% by 1984 [12]. When vaccination was introduced in the herds, both adult and young stocks were vaccinated to establish herd immunity. Thereafter, only calves were vaccinated starting at 4 weeks of age, and the authorities recommended continuing vaccination in herds for 7–10 years. Thus, the former Soviet Union was the first country to employ vaccination against bovine ringworm in a program to eliminate the disease. Successful vaccination campaigns have been reported from other European countries [73–76].
In Norway, prevalence data of herds with clinical ringworm are based on monthly reports submitted by the local veterinarians to the animal health authority. Herds are recorded once when the first case appears, and this reporting system of new infected herds has provided information about the ringworm situation since 1973 (Fig. 4) [68]. The number of new infected herds reported annually has decreased from approximately 1000 (annual incidence rate new infected herds 1.8%) before 1979 to less than 10 in 2006 (annual incidence rate 0.040%). Currently, a concerted action has been undertaken in order to focus the control measures on those herds still having problems with ringworm to eliminate the disease from these herds.
The control strategy in herds with a positive ringworm diagnosis largely follows the Russian recommendations. All non-infected cows and calves are vaccinated. Infected animals, or those with a history of ringworm, have presumably developed protective immunity and are not vaccinated. Vaccination of cows during the last 2 months of pregnancy is postponed until after calving. This program is followed by vaccination of all calves born from day one and purchased animals with no vaccination status. Duration of immunity is long lasting and revaccination is not recommended. Development of clinical lesions is prevented as well as transmission of dermatophytes to other animals and contamination of the environment. The spores may survive for several years, therefore vaccination should continue for a period of 3–5 years once started. In a program for eradication of ringworm from herds, cleaning and disinfection is recommended as additional measures and contributing to reduction in the level of contamination. Improved hygiene shortens the time needed to achieve the goal of animals without signs of ringworm and the long-term goal of eradication of ringworm from the herd.
In Sweden, a different approach was employed concerning ringworm control, in a program aiming at improving hide quality [76]. Farmers organized in the “Faultless hide scheme” agreed to vaccinate the cattle and also focus on other measures to reduce hide damage. A beneficial effect on both ringworm prevalence and quality of the hides was observed in the herds participating in the program, whereas the disease situation remained almost unchanged for those outside the scheme.
Vaccination Against Dermatophytosis in Companion Animals (Dogs, Cats and Horses)
Historical Perspective and Development Efforts
The dramatic effectiveness of vaccination programs for food and fur-bearing animals in many countries stimulated the search for an effective (and ideally, prophylactic) vaccine in companion animals. Historically, the biologics industry in Eastern Europe was active in development of vaccines for such use in horses, including the early “SP1” (Sarkisov-Petrovich-1) equine trichophytosis vaccine [15, 77]. These vaccines were never distributed widely to other countries, and faded from use as geopolitical events evolved. Notably, initial efforts often disclosed substantial host species differences in the ability of a vaccine product to protect against infection. For example, a commercial cattle vaccine based on T. verrucosum was reported to protect calves against M. canis infection, but to lack effectiveness in dogs [78]. Apparently, to stimulate long lasting and appropriate cell-mediated immunity, inactivated vaccines intended for companion animals required addition of an adjuvant. An adjuvanted vaccine formulation containing two inactivated strains of T. equinum was studied in horses [79]. The relative protection was 75% for epicutaneous challenge and 87% for challenge by commingling studies.
Only recently were efforts made to address vaccination of cats. Preliminary studies in a guinea pig model using live and inactivated vaccine formulations of two different strains of M. canis demonstrated best protection following challenge in animals vaccinated with the inactivated vaccine [80]. The vaccines were prepared according to a special protocol and stimulated DTH response; however, there was no mention of adjuvant content. The authors proposed follow-up studies in cats, but so far nothing has been published. Two experimental vaccine preparations have been studied in this host. A killed preparation of M. canis cell wall components, injected into 8–9-week-old kittens biweekly for five doses, induced both anti-dermatophyte antibody titers and cell-mediated immunity (as measured by antigen-stimulated lymphocyte blastogenesis), but these immunologic responses were not as strong as those seen in natural infection. The vaccination procedure did not confer resistance to challenge infection either via direct spore application [33] or via commingling with an infected cat [34].
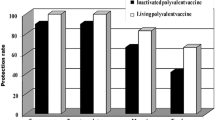
Subsequently, an experimental combined live-inactivated dermatophyte vaccine, consisting of attenuated live Trichophyton equinum plus killed components from two Microsporum strains, was tested in cats under laboratory conditions. The product was administered to kittens using several different protocols for up to 3 times at 2-week intervals. This vaccine induced a strong antibody response, but only slight and variable cell-mediated immunity. Six out of 10 cats vaccinated developed apparent, though mild, local infection with the vaccine strain at the injection site. The conferred response was not protective against direct challenge infection with M. canis, and did not provide a more rapid cure of the infection once established, but slightly reduced severity of the initial stages of the challenge infection [81]. In this same study (Fig. 5), the effectiveness of a commercially available killed vaccine product was evaluated in parallel with the experimental vaccine, and was also found to lack prophylactic activity (see below).
Immunologic response in cats induced by an experimental dermatophytosis vaccine (□) and a commercial feline vaccine (▲). Note that both vaccines induced strong antibody titers (–) by Week 12, but only slight and variable cell-mediated responses (- - -). Neither vaccine was protective against challenge infection at week 13 (arrow) via commingling with an infected cat. This study highlights the relative lack of importance of inducing a humoral immune response in feline dermatophytosis vaccines intended for prophylaxis. Data adapted from study referenced herein [80]
A commercial vaccine product (Fel-O-Vax MC-K, Fort Dodge Laboratories) consisting of killed M. canis components in adjuvant was licensed in the United States for feline use in 1994. The major indication for this product was to reduce severity of, or hasten resolution of, the clinical signs of dermatophytosis in cats, rather than for use as a prophylactic vaccine. When tested in an experimental infection model in cats, the commercial vaccine did not prevent establishment of challenge infection, although the initial severity of the infection was slightly reduced. Moreover, the vaccine did not hasten resolution of the challenge infection as compared with unvaccinated controls [81]. Anecdotal reports of field experience with the vaccine were generally not positive, with the main perception being that though it appeared to reduce clinical signs in some cats, it did not prevent infection nor eliminate the fungus from an active infection. The product was withdrawn from the market by the manufacturer in 2003 and is no longer available.
A commercially developed inactivated M. canis preparation in aluminum hydroxide adjuvant (MICANFIN, Bioveta Inc.; later called Biocan M) was tested for prophylactic efficacy in 13 cats [82]. Following two subcutaneous or intramuscular injections 14 days apart, cats were challenged by direct topical spore application. Vaccinated cats developed only minute skin changes at the challenge site, consisting of scaling and papules, with the skin returning to normal at 28 days postchallenge or less, and negative fungal cultures at that time. In contrast, unvaccinated controls developed much more severe lesions; at day 28, lesions were still present and all animals were culture positive. Thus, there was some indication that this preparation had prophylactic properties. However, the product has now been replaced on the market by a preparation that does not contain an adjuvant, hence may or may not have the same properties (see below).
Currently Available Products and Recommended Uses
Dermatophyte vaccines for use in companion animals contain either attenuated or inactivated whole fungal cells of different species. The Czech company Bioveta Inc. (Ivanovice na Hane, Czech Republic) has developed monovalent, inactivated, unadjuvanted M. canis vaccines for use in the cat (Biofel M Plus) and the dog (Biocan M Plus; a different formulation than Biocan M), and an attenuated live T. equinum vaccine intended for horses (Trichoequen) [83] (Table 1). Currently, Bioveta vaccines have market authorization in some countries mostly in Eastern Europe, but recently Biocan M has become available in Germany under the trade name RIVAC® Mikroderm® (Riemser Arzneimittel AG). It is difficult to formulate an informed opinion on the efficacy of these products, due to lack of available information in the scientific literature.
Currently, there are no published studies on the use of Biofel M Plus or Biocan M Plus for cats and dogs. Information about these products is only available from the manufacturer, claiming protection when used prophylactically and a shorter disease course when used in animals presenting signs of infection [83]. The manufacturer states that the feline vaccine can be administered to kittens at 8 weeks of age, and that immunity persists for 1 year. Puppies can be vaccinated from the age of 8 weeks, and they need two doses 10–21 days apart. Immunity is established 1 month after the last injection, and annual revaccination is recommended.
Bioveta’s equine vaccine (Trichoequen) is based on the live-attenuated mutant strain T. equinum TE-M-141 [83, 84]. Foals can be vaccinated as early as 4 months of age. Two doses at 10–16 day intervals are required. Upon epicutaneous challenge, vaccinated horses show only short-term superficial skin changes which disappear spontaneously, whereas unvaccinated controls develop more severe and disseminated disease. Some therapeutic efficacy has also been demonstrated [84]. Duration of immunity is 1 year, therefore requiring annual revaccination.
Another dermatophyte vaccine (Insol® Dermatophyton, Boehringer Ingelheim) is available in several continental European countries and in the United Kingdom, but not in the Americas or Asia [85] (Table 1). It is a polyvalent, unadjuvanted, inactivated vaccine containing fungal species T. verrucosum, T. mentagrophytes, T. sarkisovii, T. equinum, M. canis, M. canis var. distortum, M. canis var. obesum, and M. gypseum. According to the manufacturer’s literature, it is produced using a special manufacturing process that enables the inactivated microconidia to stimulate a cell-mediated immune response [85]. For treatment of active infections in horses over 5 months of age, the manufacturer’s recommended protocol is to administer two doses by deep intramuscular injection, 14 days apart. If no visible improvement occurs within 2 weeks after the second injection, a third injection may be given. For prophylactic use, two injections are given IM, 14 days apart, with repeat of the series every 9 months. Limited published investigations on the efficacy of Insol® Dermatophyton make it difficult to draw firm conclusions as to its most appropriate use. The only studies published are in horses, where the product was evaluated for therapeutic efficacy and not as a prophylactic [86, 87]. The latter studies are open, uncontrolled field studies in which horses with active infections were treated with the vaccine, and recovery monitored. It was claimed that up to 95% of horses were successfully treated, but it is unknown how many of these successful outcomes could have represented spontaneous recovery (an expected result in the majority of normal horses). Insol® Dermatophyton has also been recommended, at least in some countries, for use in dogs (over 6 weeks of age) and cats (over 10 weeks of age) as well, and even in rabbits [88]. Again, two doses, 2 weeks apart have been recommended [89]. In all species, local reactions in the form of swellings and/or pain may occur in up to 3% of vaccinated animals, and more generalized systemic malaise can occur in up to 1%. It must be emphasized that published studies on the efficacy of Insol® Dermatophyton as a prophylactic vaccine in any species are not available, nor have studies of any kind been published on its use in dogs or cats. Moreover, personal experiences and opinion expressed at a roundtable event at the Fifth World Congress of Veterinary Dermatology suggest limited efficacy [90]. Due to the lack of published information on this product, it is difficult to make specific recommendations for its use.
It appears that the goal of a widely available, truly prophylactic vaccine against dermatophytosis in dogs, cats, or horses has not been attained, but success in other species in several countries, increasing knowledge regarding the immune response to infections, and increasing globalization of manufacturing, marketing, and regulatory efforts hold promise that this goal will be eventually achieved.
Vaccination Against Dermatophytosis in Fur-Bearing Animals and Rabbits
In fur-bearing animals (including silver fox, blue fox, chinchilla, and mink) and rabbits, dermatophytosis is most often caused by T. mentagrophytes and M. canis [91–94]. Two vaccines have been used to prevent the disease in these animal species, both containing live attenuated cells of T. mentagrophytes [95, 96]. The Russian vaccine Mentavak, developed by researchers at the VIEV, Moscow [13] was used extensively in former Soviet Union in farmed foxes and rabbits [97]. A shift in the predominant dermatophyte species isolated from these animals was observed; T. mentagrophytes being most prevalent prior to the vaccination campaign and M. canis being isolated from most cases of ringworm after the campaign. The Russian vaccine was used during an outbreak of ringworm caused by T. mentagrophytes in two Swedish fox farms [98]. Adults and cubs were vaccinated by two injections and no new cases appeared after the second vaccination was completed. All cubs born the next year were vaccinated as T. mentagrophytes had been isolated from a few adult foxes and in environmental samples. No signs of ringworm were observed the following year and therefore vaccination was suspended during the next birth season. Samples collected from the environment and from animals were all negative. The authors concluded that vaccination and hygienic measures contributed to successful eradication of the disease by creating herd immunity and reducing level of environmental contamination. However, mock-vaccinated control animals were not included in this field trial and therefore the authors mentioned the need for further studies.
The vaccine Trichopelen (Bioveta, Ivanovice na Hane, Czech Republic) contains an attenuated strain of T. mentagrophytes and is indicated for use in chinchilla, rabbit, and fur-bearing animals [96] (Table 1). The vaccination schedule includes two doses by intramuscular injection with an interval of 8–12 days. Immunity is established one month after the last dose with an expected duration of minimum one year. In an experimental study in rabbits, animals vaccinated by the subcutaneous route at 11–14 days of age and those vaccinated intramuscularly at 4 months of age were all protected against epicutaneous challenge of a virulent strain of T. mentagrophytes [99]. The vaccine strain survived in the muscle for 9–10 days and hair loss and some scaling was observed at the injection site confirming some residual virulence. In an outbreak of ringworm in a large breeding colony of rabbits, all female breeding animals were vaccinated with Trichopelen protecting approximately 90% of the animals [100, 101].
Kostro [102] isolated different strains of T. mentagrophytes var. granulosum from arctic fox and examined immunological properties in guinea pig and fox models. Two of six strains stimulated strong cell-mediated immunity. Guinea pigs immunized with these two strains were well protected against challenge with a virulent strain of T. mentagrophytes.
Efficacy and safety of an experimental vaccine against M. canis infection were evaluated in silver fox and blue fox [103, 104]. The polyvalent vaccine, containing live attenuated T. equinum and inactivated cells of two strains of M. canis, was given twice intramuscularly to four- and six-week old cubs. Five weeks post last vaccination, the animals were challenged by epicutaneous inoculation of a virulent strain of M. canis. Vaccinated animals presented a transient reaction with superficial scaling 7–14 days post inoculation, whereas the controls developed typical signs of ringworm. None of the animals showed general adverse reactions. However, at the injection site, hair loss and scaling appeared 2–4 weeks post vaccination. In a field trial, 32% of the animals examined presented similar local reactions lasting for 2–6 weeks. The live vaccine component was regularly cultured from crust material at the injection site.
Impact of Vaccination
Zoonotic Situation
Dermatophytosis in animals is an important zoonotic disease transmitted to man most often by direct contact with an infected animal or indirectly from the environment contaminated by fomites from the animal. Thus, dermatophytes belonging to the zoophilic group are the most prevalent when considering transmission from animals to man [2] including M. canis, T. verrucosum, T. equinum, and others. Control and prevention of the disease in the animal reservoir is the most important measure to reduce occurrence in man. Ringworm transmitted from food and fur-bearing animals is an occupational disease of farmers and their households, veterinarians and people working in abattoirs and tanneries [105–107]. Exposure from companion animals, concerns owners of these animals and often include children [108]. Attention has also been given to the group of immunocompromised persons regarding risk of disseminated disease after exposure to dermatophytes [109–111].
The systematic vaccination campaign against cattle ringworm in the former Soviet Union has obviously contributed to a significant decrease in dermatophytosis in man caused by T. verrucosum [13]. In Norway, cattle ringworm is confined to less than 10 farms after almost 30 years of vaccination and in parallel, number of cases in the human population has decreased [112]. Today private and public diagnostic laboratories rarely isolate T. verrucosum indicating that ringworm transmitted from cattle to man is almost nonexistent. The former German Democratic Republic experienced a similar reduction in number of human cases of ringworm caused by T. verrucosum in the 1980s, and this was seen as a consequence of extensive use of vaccination in the cattle population [113]. Successful campaigns to eliminate the dermatophyte from the reservoir population clearly demonstrate the beneficial effect on the zoonotic situation. M. canis infections in companion animals are on the increase in some countries, and there are several reports on transmission to man [108, 114]. If an efficacious vaccine had been available, the experience from vaccination against cattle ringworm is likely to be applicable also in these animals.
Pelt and Hide Quality
In farms keeping fur-bearing animals, dermatophytosis is among the conditions reducing pelt quality and having serious economic consequences [115, 116]. When there is an increased risk of infection, vaccination may be a measure to avoid an outbreak. However, the importance to maintain good level of biosecurity and hygiene must be underscored. Vaccination has been used successfully in the former Soviet Union and some other Eastern European countries to prevent the disease caused by T. mentagrophytes [13].
In cattle, the hide is the most valuable by-product of the carcass. The economic significance of ringworm is related to the downgrading of hides as apparently healed skin lesions reappear after the tanning process (Fig. 6) [117]. The British Leather Confederation estimated losses in the UK leather trade industry from poor quality cattle hides to about £35 million per year of which ringworm accounted for 5% [116, 118]. In the international hide market, price setting is based on quality assessment by buyers and the level reflects prices obtained previous years. This system does not allow price differentiation between hides with and without ringworm scars. Moreover, prevalence of hides presenting ringworm lesions or damage by other causes is not recorded. A vaccination program was offered to farmers by the Swedish leather industry as one of several measures to improve hide quality [76]. Vaccination against bovine ringworm has contributed to improved quality of hides in Norway and Sweden and better prices on the European market [119, O Nafstad, personal communication]. This aspect is underscored in cost-benefit considerations of vaccination against cattle ringworm [120]. Obviously, other parameters also contribute as the zoonotic character of the disease, and in Norway, the costs related to restrictions imposed on ringworm positive herds add to the bill and justify vaccination.
Tanned cattle hide from a calf experimentally inoculated epicutaneously with a virulent strain of Trichophyton verrucosum producing a typical ringworm lesion 5 weeks after challenge. The black arrows point to the juncture between the paler, diseased hide and the normal-colored hide. The red arrow points to a defect following biopsy sampling. (Lund and Bratberg, unpublished)
Concluding Remarks
Obviously, use of live attenuated vaccines against trichophytosis in cattle and fur-bearing animals has contributed to the control or eradication of ringworm in these species, positively impacting overall herd health and hide and pelt quality, and greatly diminishing zoonotic transmission and concurrent infection in farm personnel. Despite these resounding successes, however, one must exercise caution in translating these positive results to other clinical situations. For example, in addition to prophylactic use, manufacturers also often recommend therapeutic use, and claim effects on the duration of the course of infection. In the literature, convincing data on this procedure are lacking. Importantly, very few efficacy studies (prophylactic or therapeutic) have been conducted and published in companion animals, even where there is a commercial product on the market for such use. When such efficacy studies exist, they may consist of a very small number of animals, or may be uncontrolled (and therefore difficult to interpret), or they may have been done using a preparation different from the one that eventually appeared on the market. Indeed, the evidence for efficacy of commercially available companion animal vaccines is extremely limited at this point.
Dermatophyte vaccines with the most proven success are those which contain live attenuated fungal strains. However, vaccines containing live components with residual virulence are certainly not an alternative in companion animals. Close human contact increases the risk of transfer of the vaccine strain in those cases where a lesion develops at the injection site, and may be of increased concern in households with immunosuppressed humans. There is definitely a need for well documented, safe and efficacious vaccines against dermatophytosis in companion animals. Prospects for vaccine development should be based on insight into the immune mechanisms providing protection and immunodominant dermatophyte antigens stimulating a strong DTH reaction. Some areas for future research are listed in Box 1.
References
Stenwig H. Isolation of dermatophytes from domestic animals in Norway. Nord Vet Med. 1985;37:161–9.
Stenwig H. Transmission of dermatophytes from animals to man. In: Bermann B, Wærsted A, editors. Treatment of superficial fungal infections. Oslo, Norway: Medical Products Agency, Uppsala, Sweden and Norwegian Medicines Control Authority; 1996. p. 27–32.
DeBoer DJ, Moriello KA. Dermatophytosis. In: Greene CE, editors. Infectious diseases of the dog and cat. 3rd ed. Saunders, Missouri 63146, USA; 2006. 550–65.
Radostits OM, Gay CC, Blood DC, Hinchcliff KW, editors. Dermatomycoses. In: Veterinary medicine. A textbook of the diseases of cattle, sheep, pigs, goats and horses. WB Saunders, Philadelphia, PA 2007; 10th ed. 1476–8.
Grappel SF, Bishop CT, Blank F. Immunology of dermatophytes and dermatophytosis. Bacteriol Rev. 1974;38:222–50.
Lepper AWD. Experimental bovine Trichphyton verrucosum infection: preliminary clinical, immunological and histological observations in primarily infected and reinoculated cattle. Res Vet Sci. 1972;13:105–15.
Hussin Z, Smith JMB. Vaccination procedures and the infectivity of dermatophyte lesions. Mycopathologia. 1983;81:71–6.
Calderon RA. Immunoregulation of dermatophytosis. Crit Rev Microbiol. 1989;16:339–68.
Woodfolk JA, Platts-Mills TAE. The immune response to dermatophytes. Res Immunol. 1998;149:436–45.
Smith JMB, Griffin JFT. Strategies for the development of a vaccine against ringworm. J Med Vet Mycol. 1995;33:87–91.
Kielstein P, Richter W. Versuche zur Immunprophylaxe der Rindertrichophytie. [Immunoprophylaxis of ringworm in cattle]. Arch Exper Veterinärmed. 1970;24:1205–18. (in German).
Tretiakov AD, Sarkisov AKh, Petrovich SV, Nikiforov LJ. Création de troupeaux immuns et éradication de la Trichophytose par la vaccination des animaux. [Establishment of herd immunity and eradication of trichophytosis by vaccination of animals]. Bulletin OIE. 1980;92:797–803 (in French).
Sarkisov AKh. Modern methods for control of animal dermatomycosis. Moscow: Advances in Agricultural Sciences; 1987. 181–97.
Sarkisov AKh, Petrovich SV, Nikiforov LI, Jablochnik LM, Koroljova VP. Immunization of cattle against tinea. Veterinariia. 1971;2:54–6 (in Russian).
Petrovich SV, Sarkisov Akh. Specific prophylaxis of ringworm in horses (live vaccine against T. equinum). Veterinariia. 1981;9:40–2 (in Russian).
Sarkisov AKh, Nikiforov LI. Specific prophylaxis of trichophytosis in fur animals. Veterinariia. 1981;7:37–8 (in Russian).
Stankushev Kh, Duparinova M, Kostov G, Gradinarski I. Comparative immunological studies and the determination of the epizootiological effectiveness of the Soviet vaccine LTF-130 in trichophytosis. Vet Med Nauki. 1979;16:67–73 (in Bulgarian).
Horvath Z, Gaal T. A szarvas’murha-tarlosömör elleni LTF-130 (SZU) clöcsiras vakcina Kiprobalasi eredmenyeu. [Results of a trial using the vaccine LTF-130 against cattle ringworm]. Magyar Allatorvosok Lapja. 1977;32:452–4 (in Hungarian).
Rotermund H, Franz H, Hausburg G. Erste Erfahrungen bei der Anwendung der sowjetischen Trichophytievakzine LTF-130. [First experiences with the use of the Soviet vaccine LTF-130 against trichophytosis]. Monatsh Veterinaermed. 1977;32:576–7 (in German).
Krdzalic P, Stojicevic S, Bresjanac D. Practicne mogucnosti za immunoprofilaksu trichficije goveda u industrijskom uzgoju. [Practical experiences in immunoprophylaxis against trichophytosis of cattle]. Veter-Glasnik. 1978;32:343–9 (in Serbian).
Aamodt O, Naess B, Sandvik O. Vaccination of Norwegian cattle against ringworm. Zentralblatt Veterinarmed B 1982;29:451–6.
Otcenásek M, Stros K, Komárek J, Tomsíková A, Rasková H, Hamácek F. Zkusenosti s Vakcinaci skotu proti trichfytoze pri prevenci a tlumeni dermatofytickych zoonoz. [Vaccination of cattle against trichophytosis in the prevention and control of dermatophytic zoonoses]. Vet Med (Prague). 1981;26:193–202 (in Czech).
DeBoer DJ, Moriello KA. Humoral and cellular immune responses to Microsporum canis in naturally occurring feline dermatophytosis. J Med Vet Mycol. 1993;31:121–32.
Sparkes AH, Stokes CR, Gruffydd-Jones TJ. Humoral immune responses in cats with dermatophytosis. Am J Vet Res. 1993;54:1869–73.
Pier AC, Ellis JA, Mills KW. Development of immune response to experimental bovine Trichophyton verrucosum infection. Vet Dermatol. 1993;3:131–8.
Woodfolk JA. Allergy and dermatophytes. Clin Microbiol Rev. 2005;18:30–43.
Sparkes AH. Experimental Microsporum canis infection in cats: correlation between immunological and clinical observations. J Med Vet Mycol. 1995;33:177–84.
Calderon RA, Hay RJ. Cell-mediated immunity in experimental murine dermatophytosis. II. Adoptive transfer of immunity to dermatophyte infection by lymphoid cells from donors with acute or chronic infections. Immunology. 1984;53:465–72.
Fong TAT, Mosmann TR. The role of IFN-γ in delayed-type hypersensitivity mediated Th1 clones. J Immunol. 1989;143:2887–93.
Koga T, Ishizaki H, Matsumoto T, Hori Y. In vitro release of interferon-γ by peripheral blood mononuclear cells of patients with dermatophytosis in response to stimulation with trichophytin. Br J Dermatol. 1993;128:703–4.
Koga T, Ishizaki H, Matsumoto T, Hori Y. Decreased release of interferon-γ by peripheral blood mononuclear cells of patients with chronic dermatophytosis in response to stimulation with trichophytin. Acta Dermatol Venereol. 1995;75:81–2.
Lund A, Bratberg AM, Solbakk IT. In vitro release of interferon-γ by trichophytin-stimulated whole blood cell cultures from ringworm-vaccinated and control calves experimentally inoculated with Trichpohyton verrucosum. Vet Dermatol. 2001;12:75–80.
DeBoer DJ, Moriello KA. The immune response to Microsporum canis induced by a fungal cell wall vaccine. Vet Dermatol. 1994;5:47–55.
DeBoer DJ, Moriello KA. Investigations of a killed dermatophyte cell wall vaccine against infection with Microsporum canis in cats. Res Vet Sci. 1995;59:110–3.
Elad D, Segal E. Immunogenicity in calves of a crude ribosomal fraction of Trichophyton verrucosum: a field trial. Vaccine. 1995;13:83–7.
Elad D, Segal E. Immunogenicity in guinea pigs of a crude ribosomal fraction from Microsporum canis. Vaccine. 1994;12:134–8.
Monod M, Capoccia S, Lechenne B, Saugg C, Holdom M, Jousson O. Secreted proteases from pathogenic fungi. Int J Med Microbiol. 2002;292:405–19.
Jousson O, Lechenne B, Bontems O, Capoccia S, Mignon B, Barblan J, Quadroni M, Monod M. Multiplication of an ancestral gene encoding secreted fungalysin preceded species differentiation in the dermatophytes Trichophyton and Microsporum. Microbiol. 2004;150:301–10.
Mignon B. Nouvelles recherches sur la caractérisation des facteurs de virulence de Microsporum canis. [New studies on the characterization of virulence factors in Microsporum canis]. Bull Mem Acad R Med Belg. 2005;160:270–5 (in French).
Brouta F, Descamps F, Monod M, Vermout S, Losson B, Mignon B. Humoral and cellular immune response to a Microsporum canis recombinant keratinolytic metaolloprotease (r-MEP3) from experimentally infected guinea pigs. Med Mycol. 2003;41:495–501.
Vermout SM, Brouta FD, Descamps FF, Losson BJ, Mignon BR. Evaluation of immunogenicity and protective efficacy of Microsporum canis metalloprotease subunit vaccine in guinea pigs. FEMS. 2004;40:75–80.
Descamps FF, Brouta F, Vermout SM, Willame C, Losson BJ, Mignon BR. A recombinant 31.5 kDa keratinase and a crude exo-antigen from Microsporum canis fail to protect against homologous experimental infection in guinea pigs. Vet Dermatol. 2003;14:305–12.
Woodfolk JA, Sung SSJ, Benjamin DC, Lee AJK, Platts-Mills TAE. Distinct human T cell repertoires mediate immediate and delayed-type hypersensitivity to the Trichophyton antigen Tri r 2. J Immunol. 2000;165:4379–87.
Raska M, Rybnikar A, Chumela J, Belakova J, Weigl E. Recombinant protein and DNA vaccines derived from hsp60 Trichophyton mentagrophytes control the clinical course of trichophytosis in bovine species and guinea-pigs. Mycoses. 2004;47:407–17.
Intervet, Summary of product characteristics Bovilis® Ringvac at http://www.vmd.gov.uk/espcsite/Documents/124263.DOC (retrieved 9 May 2007).
Bioveta Inc., Summary of product characteristics for Trichoben at http://www.bioveta.cz/product.asp?vyrobekid=102, and Trichoben AV at http://www.bioveta.cz/product.asp?vyrobekid=11 (retrieved 9 May 2007).
Boehringer Ingelheim Vetmedica GmbH, Summary of product characteristics Insol® Trichophyton at http://www.imb.ie/view_spc.asp?pa_number=10007%2F034%2F001 (retrieved 9 May 2007).
Rybnikář A. Cross-immunity in calves after vaccination against trichophytosis. Acta Vet Brno. 1992;61:189–94.
Pier AC, Hodges AB, Lauze JM, Raisbeck M. Experimental immunity to Microsporum canis and cross reactions with other dermatophytes of veterinary importance. J Med Vet Mycol. 1995;33:93–7.
Wawrzkiewicz K, Wawrzkiewicz J. Early immunization of calves with an inactivated vaccine against trichophytosis. Polskie Archivum Weterynaryjne. 1988;28:5–16.
Wawrzkiewicz K, Wawrzkiewicz J. An inactivated vaccine against ringworm. Comp Immunol Microbiol Infect Dis. 1992;15:31–40.
Bratberg AM, Solbakk IT, Gyllensvaan C, Bredahl LK, Lund A. Experimentelle Challenge-Studie zur Wirksamkeit einer inaktivierten und einer attenuirten Rindertrichophytie-Vakzine. [Efficacy of an inactivated and a live bovine ringworm vaccine in an experimental challenge trial]. Tierärztliche Umschau. 1999;54:519–20 (in German).
Kocik T. Evaluation of the immunogenic properties of live and killed vaccines against trichophytosis of guinea pigs and calves. Pol Arch Weter. 1982;23:95–107 (in Polish).
Hajduch M, Drabek J, Raclavsky V, Kotala V, Michalek T, Zelenkova I. Diversity among wild type and vaccinations strains of Trichophyton verrucosum investigated using random amplified polymorphic DNA analysis. Folia Biologica (Prague). 1999;45:151–6.
Rybnikář A, Chumela J, Vrzal V. Testing the protective effects of an avirulent vaccine against bovine trichophytosis. Vet Med (Prague). 1986;31:219–26 (in Czech).
Rybnikář A, Chumela J, Vrzal V, Krupka V. Immunity in cattle vaccinated against ringworm. Mycoses. 1991;34:433–6.
Gudding R, Naess B. Vaccination of cattle against ringworm caused by Trichophyton verrucosum. Am J Vet Res. 1986;47:2415–7.
Kielstein P, Wolf H, Graser Y, Buzina W, Blanz P. Zur Variabilität von Trichophyton verrucosum-Isolaten aus Impfbeständen mit Rindertrichophytie. [On the variability of Trichophyton verrucosum isolates from vaccinated herds with ringworm of cattle]. Mycoses. 1998;41(Suppl 2):58–64 (in German).
Gudding R, Næss B, Aamodt O. Immunisation against ringworm in cattle. Vet Rec. 1991;128:84–5.
Gordon PJ, Bond R. Efficacy of a live attenuated Trichophyton verrucosum vaccine for control of bovine dermatophytosis. Vet Rec. 1996;139:395–6.
Rybnikář A, Vrzal V, Chumela J. Protective efficacy of vaccines against bovine dermatophytosis after double and single vaccination. Mycoses. 1997;41:83–6.
Rybnikář A, Vrzal V, Chumela J. The minimal effective dose of vaccine against trichophytosis in heifers. Vet Med (Prague). 1991;36:593–7 (in Czech).
Törnquist M, Bendixen PH, Pehrson B. Vaccination against ringworm of calves in specialized beef production. Acta Vet Scand. 1985;26:21–9.
Naess B, Sandvik O. Early vaccination of calves against ringworm caused by Trichophyton verrucosum. Vet Rec. 1981;109:199–200.
Rybnikář A, Vrzal V, Chumela J. Vaccination of young calves against trichphytosis. Acta Vet Brno. 1993;62:55–61.
Bratberg AM, Solbakk IT, Lund A, Bredahl LK. Sicherheit und Wirksamkeit des Impfstoffes Permavax®–Tricho/N gegen bovine Trichophytie bei neugeborenen Kälbern. [Safety and efficacy of the vaccine Premavax®-Tricho/N against trichophytosis in newborn calves]. Tierärztliche Umschau. 1998;53:686 (in German).
Rybnikář A, Chumela J, Vrzal V. Immunisation of pregnant cows against ringworm and its effect on the progeny. Acta Vet Brno. 2001;70:421–3.
Gudding R, Lund A. Immunoprophylaxis of bovine dermatophytosis. Can Vet J. 1995;36:302–6.
Siesenop U, Böhm KH, Brandenbusemeyer E, Conrad P. Studies of growth, spore-forming ability and virulence of the vaccine strain TV-M-310 of the vaccine Bioveta against ringworm. Mycoses. 1994;37:371–6.
Lund A, Bratberg AM, Evensen Ø. Cell recruitment in skin in the course of an experimental infection with Trichophyton verrucosum in a vaccinated and a non-vaccinated calf. In: Kwochka KW, Willemse T, Tscharner Cv. Advances in Veterinary Dermatology. Butterworth-Heinemann; 1998;3:271–81.
Panin AN, Manoyan MG, Letyagin KP, Sarkisov KA. Prophylaxis and therapy of trichophytosis of animals by the vaccine “VERMET” [abstract]. Vet Dermatol. 2000;11(Suppl 1):40.
Sarkisov Akh. Prophylaxie spécifique de la Trichophytose des jeunes bovins. [Specific prophylaxis of trichophytosis in young cattle]. Bulletin OIE. 1976;85:481–8 (in French).
Kielstein P. Systematic control of dermatophytosis profunda of cattle in the former GDR. Mycoses. 1990;33:575–9.
Rybnikář A, Vrzal V, Chumela J, Hejtmánek M, Weigl E. Vaccination of cattle against trichophytosis using the Czech vaccines. J Mycol Méd. 1996;6:93–4.
Spanoghe L, Kuiper JD, Brethouwer AH. Een onderzoek naar de werkzaamheid van het trichofytie-vaccin LTF 130 onder praktykomstandigheden. [A study on efficacy of the trichophytosis vaccine LTF 130 under practical conditions]. Tijdschri Diergeneeskd. 1985;110:1011–4 (in Dutch).
Gyllensvaan C, Plym Forshell K, Törnquist M. Projekt Felfri hud - en lägesrapport. [The Faultless hide scheme—status report]. Sv Vet Tidn. 1992;44:423–4 (in Swedish).
Mackenzie DWR, Loeffler W, Mantovani A, Fujikura T. Guidelines for the diagnosis, prevention, and control of dermatophytoses in man and animals. Geneva: World Health Organization; 1986.
Rybnikář A, Vrzal V, Chumela J. Vaccination of dogs and calves against Microsporum canis. Acta Vet Brno. 1996;65:161–4.
Pier AC, Zancanella PJ. Immunization of horses against dermatophytosis caused by Trichophyton equinum. Equine Practice. 1993;15:23–7.
Wawrzkiewicz K, Ziolkowska G. Specific immunoprophylaxis in Microsporum canis infection in guinea pigs. J Mycol Méd. 1996;6:56–62.
DeBoer DJ, Moriello KA, Blum JL, Volk LM, Bredahl LK. Safety and immunologic effects after inoculation of inactivated and combined live-inactivated dermatophytosis vaccines in cats. Am J Vet Res. 2002;63:1532–7.
Rybnikář A, Vrzal V, Chumela J, Petras J. Immunization of cats against Microsporum canis. Acta Vet Brno. 1997;66:177–81.
Bioveta Inc., Summary of product characteristics for Biofel M Plus at http://www.bioveta.cz/product.asp?vyrobekid=114, Biocan M Plus at http://www.bioveta.cz/product.asp?vyrobekid=142, and Trichoequen at http://www.bioveta.cz/product.asp?vyrobekid=103 (Retrieved 25 June 2007).
Rybnikář A, Chumela J, Vrzal V, Lysak J, Petras J. Vaccination of horses against trichophytosis. Acta Vet Brno. 1991;60:165–9.
Boehringer Ingelheim Vetmedica GmbH, Summary of product characteristics Insol® Dermatophyton at http://www.boehringer-ingelheim.com/corporate/products/animal_health_horse_02.htm (retrieved 17 April 2007).
Karle J, Steller U, Fenner A. Kontrollierte Blindstudie zur Prüfung der Wirksamkeit des inaktivierten Impfstoffes Insol® Dermatophyton bei künstlich infizierten Pferden. [Controlled blind study to prove the efficacy of the inactivated vaccine Insol® Dermatophyton in artificially infected horses]. Pferdeheilkunde. 2002;18:625–8 (in German).
Fenner A, Karle J. Therapeutische Impfung gegen Dermatophytosen beim Pferd mit Insol® Dermatophyton. [Therapeutic vaccination against dermatophytosis in horses with Insol® Dermatophyton]. Praktischer Tierarzt. 81:7, 574–8 (in German).
Descamps F, Brouta F, Losson B, Mignon B. Perspectives de vaccination anti-dermatophytique chez les carnivores domestiques [Perspectives on anti-dermatophytic vaccination in domestic carnivores]. Ann Med Vet. 2001;145:178–82 (in French).
Veterinary Compendium of Switzerland, Insol® Dermatophyton, at http://www-vetpharm.uzh.ch/TAK/00000000/00001500.VAK (retrieved 20 April 2007).
DeBoer DJ, Carlotti DN. Workshop report on anti-fungal therapy. In: Hiller A, Foster AP, Kwochka K, editors. Advances in veterinary dermatology, volume 5. Oxford: Blackwell; 2005. p. 306–11.
Hagen KW, Gorham JR. Dermatomycoses in fur animals: chinchilla, ferret, mink and rabbit. Vet Med Small Anim Clin. 1972;67:43–8.
Böhm KH, Loliger C. Die Verbreitung von Dermatophyten bei Pelztieren (Nerz und Chinchilla). [The distribution of dermatophytes in fur animals (mink and chinchilla)]. Zentralbl Veterinarmed B. 1969;16:775–83 (in German).
Janovitz EB, Long GG. Dermatomycosis in ranch foxes. J Am Vet Med Assoc. 1984;185:1393–4.
Torres-Rodriguez JM, Dronda MA, Rosell J, Madrenys N. Incidence of dermatophytosis in rabbit farms in Catalonia, Spain, and its repercussion on human health. Eur J Epidemiol. 1992;8:326–9.
Sarkisov AKh, Nikiforov LI. Specific prophylaxis of Trichophyton infection in furbearing animals (silver fox and arctic fox). Bull VIEV. 1981;42:32–4 (in Russian).
Bioveta Inc., Summary of product characteristics for Trichopelen at http://www.bioveta.cz/product.asp?vyrobekid=43 (retrieved 25 June 2007).
Nikiforov LI, Chuchina GV. Dynamics of dermatophyte species among furbearing animals and rabbits. Veterinariia. 1989;1:38 (in Russian).
Englund L, Mattson R, Berndtson LT. Possible effect of vaccination against Trichophyton mentagrophytes infection in a Swedish fox farm. Acta Vet Scand. 1990;31:121–3.
Rybnikář A, Chumela J, Vrzal V, Nepeřený J. Vaccination of rabbits against trichophytosis – an experimental study. Acta Vet Brno. 1998;67:121–5.
Rybnikář A, Chumela J, Vrzal V. Ringworm in furbearing animals. Veterinářstvi. 1993;43:390–1 (in Czech).
Rybnikář A, Vrzal V, Chumela J, Kovář S. Ringworm in a large breeding colony of rabbits. Veterinářstvi. 1995;45:120–1 (in Czech).
Kostro K. Immunogenicity of Trichophyton mentagrophytes isolated from arctic foxes with ringworm. Pol J Vet Sci. 2004;7:15–20.
Bredahl LK, Bratberg AM, Solbakk IT, Lund A. Efficacy of an experimental Microsporum canis vaccine in farmed foxes. Vet Dermatol. 2000;11(suppl 1):39 (Scientific abstracts, 4th World Congress of Veterinary Dermatology, San Francisco).
Bredahl LK, Bratberg AM, Solbakk IT, Lund A. Safety of an experimental Microsporum canis vaccine in farmed foxes. Vet Dermatol. 2000;11(suppl 1):45 (Scientific abstracts, 4th World Congress of Veterinary Dermatology, San Francisco).
Simaljakova M, Buchvald J, Olexova B. Microsporum canis-Infektion beim Kaninchen mit Ubertragung auf den Menschen. [Microsporum canis infection in rabbits and its transmission to humans]. Mycoses. 1989;32:93–6 (in German).
Wabacha JK, Gitau GK, Bebora LC, Bwanga CO, Wamuri ZM, Mbithi PMF. Occurrence of dermatomycosis (ringworm) due to Trichophyton verrucosum in dairy calves and its spread to animal attendants. J S Afr Vet Ass. 1998;69:172–3.
Ming PX, Xia YL, Bulmer GS. Outbreak of Trichophyton verrucosum in China transmitted from cows to humans. Mycopathologia. 2006;161:225–8.
Lunder M, Lunder B. Is Microsporum canis infection about to become a serious dermatological problem? Dermatology. 1992;184:87–9.
Aly R, Berger T. Common superficial fungal infections in patients with AIDS. Clin Infect Dis. 1996;22(Suppl 2):S128–32.
Bournerias I, De Chauvin MF, Datry A, Chambrette I, Carriere J, Devidas A, Blanc F. Unusual Microsporum canis infections in adult HIV patients. J Am Acad Dermatol. 1996;35:808–10.
Porro AM, Yoshioka MC, Kaminski SK, Palmeira Mdo C, Fischman O, Alchorne MM. Disseminated dermatophytosis caused by Microsporum gypseum in two patients with acquired immunodeficiency syndrome. Mycopathologia. 1997;137:9–12.
Sandven P. Epidemiology of dermatophyte infections. In: Bermann B, Wærsted A, editors. Treatment of superficial fungal infections. Norway, Uppsala and Oslo: Medical Products Agency, Sweden and Norwegian Medicines Control Authority; 1996. p. 27–32.
Seebacher C. Epidemiologie, Klinik und Therapie von Dermatomykosen durch zoophile Dermatophyten. [Epidemiology, clinic and treatment of dermatomycosis caused by zoophilic dermatophytes]. Mycoses. 2000;43 (Suppl 1):4–7 (in German).
Moriello KA. Review of zoonotic skin diseases of dogs and cats. Animal Res Rev. 2004;4:157–68.
Bildfell RJ, Hedstrom OR, Dearing PL. Outbreak of dermatophytosis in farmed mink in the USA. Vet Rec. 2004;155:746–8.
Haab C, Bertschinger HU, Rotz Av. Epidemiologie der Trichophytie beim Mastkalb im Hinblick auf die Verhütung von Lederschäden. [Epidemiology of ringworm in veal calves with regard to prevention of leather defects]. Schweizer Archiv für Tierheilkunde. 1994;136:217–26 (in German).
Coles GC. How to improve hide and pelt quality. J Soc Leather Technol Chem. 1996;80:39–40.
Anonymous. New ringworm vaccine. Leather 1993;195:16.
Bredahl L, Carlsson J, Gyllensvaan C. Ringorm hos nötkreatur–klinik, bekämpande och betydelse. [Cattle ringworm–clinic, control and significance]. Sv Vet Tidn. 1998;50:413–8 (in Swedish).
Gudding R. Cost-benefit considerations of vaccination against ringworm in cattle. Acta Vet Scand. 1996;90(Suppl l):67–8.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lund, A., DeBoer, D.J. Immunoprophylaxis of Dermatophytosis in Animals. Mycopathologia 166, 407–424 (2008). https://doi.org/10.1007/s11046-008-9111-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11046-008-9111-6