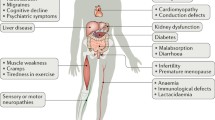
Abstract
Mitochondria play a pivotal role in mammalian cell metabolism, hosting a number of important biochemical pathways including oxidative phosphorylation. As might be expected from this fundamental contribution to cell function, abnormalities of mitochondrial metabolism are a common cause of human disease. Primary mutations of mitochondrial DNA result in a diverse group of disorders often collectively referred to as the mitochondrial encephalomyopathies. Perhaps more importantly in numerical terms are those neurodegenerative diseases caused by mutations of nuclear genes encoding mitochondrial proteins. Finally there are mitochondrial abnormalities induced by secondary events e.g. oxidative stress that may contribute to senescence, and environmental toxins that may cause disease either alone or in combination with a genetic predisposition.
Similar content being viewed by others
References
Leonard JV, Schapira AH (2000) Mitochondrial respiratory chain disorders I: mitochondrial DNA defects. Lancet 355:299–304. doi:10.1016/S0140-6736(99)05225-3
Leonard JV, Schapira AH (2000) Mitochondrial respiratory chain disorders II: neurodegenerative disorders and nuclear gene defects. Lancet 355:389–394. doi:10.1016/S0140-6736(99)05226-5
Schapira AH (2006) Mitochondrial disease. Lancet 368:70–82. doi:10.1016/S0140-6736(06)68970-8
Morgan-Hughes JA, Sweeney MG, Cooper JM, Hammans SR, Brockington M, Schapira AH, Harding AE, Clark JB (1995) Mitochondrial DNA (mtDNA) diseases: correlation of genotype to phenotype. Biochim Biophys Acta 1271:135–140
Morgan-Hughes JA, Schapira AH, Cooper JM, Clark JB (1988) Molecular defects of NADH-ubiquinone oxidoreductase (complex I) in mitochondrial diseases. J Bioenerg Biomembr 20:365–382. doi:10.1007/BF00769638
Berenberg RA, Pellock JM, DiMauro S et al (1977) Lumping or splitting? “Ophthalmoplegia-plus” or Kearns-Sayre syndrome? Ann Neurol 1:37–54. doi:10.1002/ana.410010104
Holt IJ, Harding AE, Morgan-Hughes JA (1988) Deletions of muscle mitochondrial DNA in patients with mitochondrial myopathies. Nature 331:717–719. doi:10.1038/331717a0
Holt IJ, Harding AE, Cooper JM et al (1989) Mitochondrial myopathies: clinical and biochemical features of 30 patients with major deletions of muscle mitochondrial DNA. Ann Neurol 26:699–708. doi:10.1002/ana.410260603
Moraes CT, DiMauro S, Zeviani M et al (1989) Mitochondrial DNA deletions in progressive external ophthalmoplegia and Kearns-Sayre syndrome. N Engl J Med 320:1293–1299
Zeviani M, Moraes CT, DiMauro S et al (1998) Deletions of mitochondrial DNA in Kearns-Sayre syndrome. Neurology 51:1525
Hammans SR, Sweeney MG, Brockington M et al (1991) Mitochondrial encephalopathies: molecular genetic diagnosis from blood samples. Lancet 337:1311–1313. doi:10.1016/0140-6736(91)92981-7
De Volder A, Ghilain S, de Barsy T et al (1988) Brain metabolism in mitochondrial encephalomyopathy: a PET study. J Comput Assist Tomogr 12:854–857. doi:10.1097/00004728-198809010-00024
Kobayashi Y, Momoi MY, Tominaga K et al (1990) A point mutation in the mitochondrial tRNA(Leu)(UUR) gene in MELAS (mitochondrial myopathy, encephalopathy, lactic acidosis and stroke-like episodes). Biochem Biophys Res Commun 173:816–822. doi:10.1016/S0006-291X(05)80860-5
Hammans SR, Sweeney MG, Brockington M et al (1993) The mitochondrial DNA transfer RNA(Lys)A–>G(8344) mutation and the syndrome of myoclonic epilepsy with ragged red fibres (MERRF). Relationship of clinical phenotype to proportion of mutant mitochondrial DNA. Brain 116(Pt 3):617–632. doi:10.1093/brain/116.3.617
Berkovic SF, Carpenter S, Evans A et al (1989) Myoclonus epilepsy and ragged-red fibres (MERRF). 1. A clinical, pathological, biochemical, magnetic resonance spectrographic and positron emission tomographic study. Brain 112(Pt 5):1231–1260. doi:10.1093/brain/112.5.1231
Shoffner JM, Lott MT, Lezza AM, Seibel P et al (1990) Myoclonic epilepsy and ragged-red fiber disease (MERRF) is associated with a mitochondrial DNA tRNA(Lys) mutation. Cell 61:931–937. doi:10.1016/0092-8674(90)90059-N
Zeviani M, Amati P, Bresolin N et al (1991) Rapid detection of the A—G(8344) mutation of mtDNA in Italian families with myoclonus epilepsy and ragged-red fibers (MERRF). Am J Hum Genet 48:203–211
Holt IJ, Harding AE, Petty RK et al (1990) A new mitochondrial disease associated with mitochondrial DNA heteroplasmy. Am J Hum Genet 46:428–433
Tsairis P, Engel W, Kark P (1973) Familial Myoclonid epilepsy syndrome associated with skeletal muscle mitochondrial abnormalities. Neurology 23:408
Takeda S, Wakabayashi K, Ohama E et al (1988) Neuropathology of myoclonus epilepsy associated with ragged-red fibers (Fukuhara’s disease). Acta Neuropathol 75:433–440. doi:10.1007/BF00687129
Ortiz RG, Newman NJ, Shoffner JM, Kaufman AE, Koontz DA, Wallace DC (1993) Variable retinal and neurologic manifestations in patients harboring the mitochondrial DNA 8993 mutation. Arch Ophthalmol 111:1525–1530
Fryer A, Appleton R, Sweeney MG et al (1994) Mitochondrial DNA 8993 (NARP) mutation presenting with a heterogeneous phenotype including ‘cerebral palsy’. Arch Dis Child 71:419–422
Makela-Bengs P, Suomalainen A, Majander A et al (1995) Correlation between the clinical symptoms and the proportion of mitochondrial DNA carrying the 8993 point mutation in the NARP syndrome. Pediatr Res 37:634–639. doi:10.1203/00006450-199505000-00014
Puddu P, Barboni P, Mantovani V et al (1993) Retinitis pigmentosa, ataxia, and mental retardation associated with mitochondrial DNA mutation in an Italian family. Br J Ophthalmol 77:84–88. doi:10.1136/bjo.77.2.84
Uziel G, Moroni I, Lamantea E et al (1997) Mitochondrial disease associated with the T8993G mutation of the mitochondrial ATPase 6 gene: a clinical, biochemical, and molecular study in six families. J Neurol Neurosurg Psychiatry 63:16–22
Chowers I, Lerman-Sagie T, Elpeleg ON et al (1999) Cone and rod dysfunction in the NARP syndrome. Br J Ophthalmol 83:190–193
DiMauro S, De Vivo DC (1996) Genetic heterogeneity in Leigh syndrome. Ann Neurol 40:5–7. doi:10.1002/ana.410400104
van Erven PM, Gabreels FJ, Ruitenbeek W, Renier WO, Fischer JC (1987) Mitochondrial encephalomyopathy. Association with an NADH dehydrogenase deficiency. Arch Neurol 44:775–778
Willems JL, Monnens LA, Trijbels JM, Veerkamp JH, Meyer AE, van Dam K, van Haelst U (1977) Leigh’s encephalomyelopathy in a patient with cytochrome c oxidase deficiency in muscle tissue. Pediatrics 60:850–857
Van Coster R, Lombres A, De Vivo DC, Chi TL, Dodson WE, Rothman S, Orrechio EJ, Grover W, Berry GT, Schwartz JF (1991) Cytochrome c oxidase-associated Leigh syndrome: phenotypic features and pathogenetic speculations. J Neurol Sci 104:97–111. doi:10.1016/0022-510X(91)90222-S
Kretzschmar HA, DeArmond SJ, Koch TK, Patel MS, Newth CJ, Schmidt KA, Packman S (1987) Pyruvate dehydrogenase complex deficiency as a cause of subacute necrotizing encephalopathy (Leigh disease). Pediatrics 79:370–373
Baumgartner ER, Suormala TM, Wick H, Probst A, Blauenstein U, Bachmann C, Vest M (1989) Biotinidase deficiency: a cause of subacute necrotizing encephalomyelopathy (Leigh syndrome). Report of a case with lethal outcome. Pediatr Res 26:260–266. doi:10.1203/00006450-198909000-00021
Matthews PM, Marchington DR, Squier M, Land J, Brown RM, Brown GK (1993) Molecular genetic characterization of an X-linked form of Leigh’s syndrome. Ann Neurol 33:652–655. doi:10.1002/ana.410330616
Vazquez-Memije ME, Shanske S, Santorelli FM, Kranz-Eble P, Davidson E, DeVivo DC, DiMauro S (1996) Comparative biochemical studies in fibroblasts from patients with different forms of Leigh syndrome. J Inherit Metab Dis 19:43–50. doi:10.1007/BF01799347
Loeffen J, Smeitink J, Triepels R, Smeets R, Schuelke M, Sengers R, Trijbels F, Hamel B, Mullaart R, van den HL (1998) The first nuclear-encoded complex I mutation in a patient with Leigh syndrome. Am J Hum Genet 63:1598–1608. doi:10.1086/302154
Bourgeron T, Rustin P, Chretien D, Birch-Machin M, Bourgeois M, Viegas-Pequignot E, Munnich A, Rotig A (1995) Mutation of a nuclear succinate dehydrogenase gene results in mitochondrial respiratory chain deficiency. Nat Genet 11:144–149. doi:10.1038/ng1095-144
Tiranti V, Hoertnagel K, Carrozzo R, Galimberti C, Munaro M, Granatiero M, Zelante L, Gasparini P, Marzella R, Rocchi M, Bayona-Bafaluy MP, Enriquez JA, Uziel G, Bertini E, Dionisi-Vici C, Franco B, Meitinger T, Zeviani M (1998) Mutations of SURF-1 in Leigh disease associated with cytochrome c oxidase deficiency. Am J Hum Genet 63:1609–1621. doi:10.1086/302150
Zhu Z, Yao J, Johns T, Fu K, De Bie I, Macmillan C, Cuthbert AP, Newbold RF, Wang J, Chevrette M, Brown GK, Brown RM, Shoubridge EA (1998) SURF1, encoding a factor involved in the biogenesis of cytochrome c oxidase, is mutated in Leigh syndrome. Nat Genet 20:337–343. doi:10.1038/3804
Sue CM, Karadimas C, Checcarelli N, Tanji K, Papadopoulou LC, Pallotti F, Guo FL, Shanske S, Hirano M, De Vivo DC, Van Coster R, Kaplan P, Bonilla E, DiMauro S (2000) Differential features of patients with mutations in two COX assembly genes, SURF-1 and SCO2. Ann Neurol 47:589–595. doi:10.1002/1531-8249(200005)47:5<589::AID-ANA6>3.0.CO;2-D
Ciafaloni E, Santorelli FM, Shanske S, Deonna T, Roulet E, Janzer C, Pescia G, DiMauro S (1993) Maternally inherited Leigh syndrome. J Pediatr 122:419–422. doi:10.1016/S0022-3476(05)83431-6
Santorelli FM, Shanske S, Macaya A, DeVivo DC, DiMauro S (1993) The mutation at nt 8993 of mitochondrial DNA is a common cause of Leigh’s syndrome. Ann Neurol 34:827–834. doi:10.1002/ana.410340612
de Vries DD, van Engelen BG, Gabreels FJ, Ruitenbeek W, van Oost BA (1993) A second missense mutation in the mitochondrial ATPase 6 gene in Leigh’s syndrome. Ann Neurol 34:410–412. doi:10.1002/ana.410340319
Santorelli FM, Shanske S, Jain KD, Tick D, Schon EA, DiMauro S (1994) A T-->C mutation at nt 8993 of mitochondrial DNA in a child with Leigh syndrome. Neurology 44:972–974
Cox GB, Fimmel AL, Gibson F, Hatch L (1986) The mechanism of ATP synthase: a reassessment of the functions of the b and a subunits. Biochim Biophys Acta 849:62–69. doi:10.1016/0005-2728(86)90096-4
Rahman S, Blok RB, Dahl HH, Danks DM, Kirby DM, Chow CW, Christodoulou J, Thorburn DR (1996) Leigh syndrome: clinical features and biochemical and DNA abnormalities. Ann Neurol 39:343–351. doi:10.1002/ana.410390311
Yamamoto M, Clemens PR, Engel AG (1991) Mitochondrial DNA deletions in mitochondrial cytopathies: observations in 19 patients. Neurology 41:1822–1828
Yamadori I, Kurose A, Kobayashi S, Ohmori M, Imai T (1992) Brain lesions of the Leigh-type distribution associated with a mitochondriopathy of Pearson’s syndrome: light and electron microscopic study. Acta Neuropathol 84:337–341. doi:10.1007/BF00227830
Morris AA, Taanman JW, Blake J, Cooper JM, Lake BD, Malone M, Love S, Clayton PT, Leonard JV, Schapira AH (1998) Liver failure associated with mitochondrial DNA depletion. J Hepatol 28:556–563. doi:10.1016/S0168-8278(98)80278-X
Campuzano V, Montermini L, Molto MD, Pianese L, Cossee M, Cavalcanti F, Monros E, Rodius F, Duclos F, Monticelli A, Zara F, Canizares J, Koutnikova H, Bidichandani SI, Gellera C, Brice A, Trouillas P, De Michele G, Filla A, De Frutos R, Palau F, Patel PI, Di Donato S, Mandel JL, Cocozza S, Koenig M, Pandolfo M (1996) Friedreich’s ataxia: autosomal recessive disease caused by an intronic GAA triplet repeat expansion. Science 271:1423–1427. doi:10.1126/science.271.5254.1423
Pook MA, Al Mahdawi SA, Thomas NH, Appleton R, Norman A, Mountford R, Chamberlain S (2000) Identification of three novel frameshift mutations in patients with Friedreich’s ataxia. J Med Genet 37:E38. doi:10.1136/jmg.37.11.e38
Foury F, Cazzalini O (1997) Deletion of the yeast homologue of the human gene associated with Friedreich’s ataxia elicits iron accumulation in mitochondria. FEBS Lett 411:373–377. doi:10.1016/S0014-5793(97)00734-5
Koutnikova H, Campuzano V, Foury F, Dolle P, Cazzalini O, Koenig M (1997) Studies of human, mouse and yeast homologues indicate a mitochondrial function for frataxin. Nat Genet 16:345–351. doi:10.1038/ng0897-345
Puccio H, Simon D, Cossee M, Criqui-Filipe P, Tiziano F, Melki J, Hindelang C, Matyas R, Rustin P, Koenig M (2001) Mouse models for Friedreich ataxia exhibit cardiomyopathy, sensory nerve defect and Fe–S enzyme deficiency followed by intramitochondrial iron deposits. Nat Genet 27:181–186. doi:10.1038/84818
Bradley JL, Blake JC, Chamberlain S, Thomas PK, Cooper JM, Schapira AH (2000) Clinical, biochemical and molecular genetic correlations in Friedreich’s ataxia. Hum Mol Genet 9:275–282. doi:10.1093/hmg/9.2.275
Rotig A, de Lonlay P, Chretien D, Foury F, Koenig M, Sidi D, Munnich A, Rustin P (1997) Aconitase and mitochondrial iron-sulphur protein deficiency in Friedreich ataxia. Nat Genet 17:215–217. doi:10.1038/ng1097-215
Melov S, Coskun P, Patel M, Tuinstra R, Cottrell B, Jun AS, Zastawny TH, Dizdaroglu M, Goodman SI, Huang TT, Miziorko H, Epstein CJ, Wallace DC (1999) Mitochondrial disease in superoxide dismutase 2 mutant mice. Proc Natl Acad Sci USA 96:846–851. doi:10.1073/pnas.96.3.846
Tabrizi SJ, Workman J, Hart PE, Mangiarini L, Mahal A, Bates G, Cooper JM, Schapira AH (2000) Mitochondrial dysfunction and free radical damage in the Huntington R6/2 transgenic mouse. Ann Neurol 47:80–86. doi:10.1002/1531-8249(200001)47:1<80::AID-ANA13>3.0.CO;2-K
Kemp GJ, Taylor DJ, Thompson CH, Hands LJ, Rajagopalan B, Styles P, Radda GK (1993) Quantitative analysis by 31P magnetic resonance spectroscopy of abnormal mitochondrial oxidation in skeletal muscle during recovery from exercise. NMR Biomed 6:302–310. doi:10.1002/nbm.1940060504
Lodi R, Rajagopalan B, Blamire AM, Cooper JM, Davies CH, Bradley JL, Styles P, Schapira AH (2001) Cardiac energetics are abnormal in Friedreich ataxia patients in the absence of cardiac dysfunction and hypertrophy: an in vivo 31P magnetic resonance spectroscopy study. Cardiovasc Res 52:111–119. doi:10.1016/S0008-6363(01)00357-1
Lodi R, Cooper JM, Bradley JL, Manners D, Styles P, Taylor DJ, Schapira AH (1999) Deficit of in vivo mitochondrial ATP production in patients with Friedreich ataxia. Proc Natl Acad Sci USA 96:11492–11495. doi:10.1073/pnas.96.20.11492
Cooper JM, Schapira AH (2007) Friedreich’s ataxia: coenzyme Q10 and vitamin E therapy. Mitochondrion 7(Suppl):S127–S135. doi:10.1016/j.mito.2007.04.001
Lodi R, Hart PE, Rajagopalan B, Taylor DJ, Crilley JG, Bradley JL, Blamire AM, Manners D, Styles P, Schapira AH, Cooper JM (2001) Antioxidant treatment improves in vivo cardiac and skeletal muscle bioenergetics in patients with Friedreich’s ataxia. Ann Neurol 49:590–596. doi:10.1002/ana.1001
Hart PE, Lodi R, Rajagopalan B, Bradley JL, Crilley JG, Turner C, Blamire AM, Manners D, Styles P, Schapira AH, Cooper JM (2005) Antioxidant treatment of patients with Friedreich ataxia: four-year follow-up. Arch Neurol 62:621–626. doi:10.1001/archneur.62.4.621
Thomas PK, Cooper JM, King RH, Workman JM, Schapira AH, Goss-Sampson MA, Muller DP (1993) Myopathy in vitamin E deficient rats: muscle fibre necrosis associated with disturbances of mitochondrial function. J Anat 183(Pt 3):451–461
Mordente A, Martorana GE, Minotti G, Giardina B (1998) Antioxidant properties of 2, 3-dimethoxy-5-methyl-6-(10-hydroxydecyl)-1, 4-benzoquinone (idebenone). Chem Res Toxicol 11:54–63. doi:10.1021/tx970136j
Gutzmann H, Hadler D (1998) Sustained efficacy and safety of idebenone in the treatment of Alzheimer’s disease: update on a 2-year double-blind multicentre study. J Neural Transm Suppl 54:301–310
Ranen NG, Peyser CE, Coyle JT, Bylsma FW, Sherr M, Day L, Folstein MF, Brandt J, Ross CA, Folstein SE (1996) A controlled trial of idebenone in Huntington’s disease. Mov Disord 11:549–554. doi:10.1002/mds.870110510
Hausse AO, Aggoun Y, Bonnet D, Sidi D, Munnich A, Rotig A, Rustin P (2002) Idebenone and reduced cardiac hypertrophy in Friedreich’s ataxia. Heart 87:346–349. doi:10.1136/heart.87.4.346
Schapira AH, Cooper JM, Dexter D, Jenner P, Clark JB, Marsden CD (1989) Mitochondrial complex I deficiency in Parkinson’s disease. Lancet 1:1269. doi:10.1016/S0140-6736(89)92366-0
Schapira AH, Cooper JM, Dexter D, Clark JB, Jenner P, Marsden CD (1990) Mitochondrial complex I deficiency in Parkinson’s disease. J Neurochem 54:823–827. doi:10.1111/j.1471-4159.1990.tb02325.x
Schapira AH (2007) Mitochondrial dysfunction in Parkinson’s disease. Cell Death Differ 14:1261–1266. doi:10.1038/sj.cdd.4402160
Schapira AH (2008) Mitochondria in the aetiology and pathogenesis of Parkinson’s disease. Lancet Neurol 7:97–109. doi:10.1016/S1474-4422(07)70327-7
Orth M, Schapira AH (2002) Mitochondrial involvement in Parkinson’s disease. Neurochem Int 40:533–541. doi:10.1016/S0197-0186(01)00124-3
Mann VM, Cooper JM, Daniel SE, Srai K, Jenner P, Marsden CD, Schapira AH (1994) Complex I, iron, and ferritin in Parkinson’s disease substantia nigra. Ann Neurol 36:876–881. doi:10.1002/ana.410360612
Schapira AH, Cooper JM, Morgan-Hughes JA, Patel SD, Cleeter MJ, Ragan CI, Clark JB (1988) Molecular basis of mitochondrial myopathies: polypeptide analysis in complex-I deficiency. Lancet 1:500–503. doi:10.1016/S0140-6736(88)91296-2
Schapira AH, Mann VM, Cooper JM, Dexter D, Daniel SE, Jenner P, Clark JB, Marsden CD (1990) Anatomic and disease specificity of NADH CoQ1 reductase (complex I) deficiency in Parkinson’s disease. J Neurochem 55:2142–2145. doi:10.1111/j.1471-4159.1990.tb05809.x
Cooper JM, Daniel SE, Marsden CD, Schapira AH (1995) L-dihydroxyphenylalanine and complex I deficiency in Parkinson’s disease brain. Mov Disord 10:295–297. doi:10.1002/mds.870100311
Gu M, Gash MT, Cooper JM, Wenning GK, Daniel SE, Quinn NP, Marsden CD, Schapira AH (1997) Mitochondrial respiratory chain function in multiple system atrophy. Mov Disord 12:418–422. doi:10.1002/mds.870120323
Schapira AH (1994) Evidence for mitochondrial dysfunction in Parkinson’s disease—a critical appraisal. Mov Disord 9:125–138. doi:10.1002/mds.870090202
Taylor DJ, Krige D, Barnes PR, Kemp GJ, Carroll MT, Mann VM, Cooper JM, Marsden CD, Schapira AH (1994) A 31P magnetic resonance spectroscopy study of mitochondrial function in skeletal muscle of patients with Parkinson’s disease. J Neurol Sci 125:77–81. doi:10.1016/0022-510X(94)90245-3
Parker WD Jr, Boyson SJ, Parks JK (1989) Abnormalities of the electron transport chain in idiopathic Parkinson’s disease. Ann Neurol 26:719–723. doi:10.1002/ana.410260606
Krige D, Carroll MT, Cooper JM, Marsden CD, Schapira AH (1992) Platelet mitochondrial function in Parkinson’s disease. The Royal Kings and Queens Parkinson Disease Research Group. Ann Neurol 32:782–788. doi:10.1002/ana.410320612
Mann VM, Cooper JM, Schapira AH (1992) Quantitation of a mitochondrial DNA deletion in Parkinson’s disease. FEBS Lett 299:218–222. doi:10.1016/0014-5793(92)80118-Z
Kraytsberg Y, Kudryavtseva E, McKee AC, Geula C, Kowall NW, Khrapko K (2006) Mitochondrial DNA deletions are abundant and cause functional impairment in aged human substantia nigra neurons. Nat Genet 38:518–520. doi:10.1038/ng1778
Swerdlow RH, Parks JK, Miller SW, Tuttle JB, Trimmer PA, Sheehan JP, Bennett JP Jr, Davis RE, Parker WD Jr (1996) Origin and functional consequences of the complex I defect in Parkinson’s disease. Ann Neurol 40:663–671. doi:10.1002/ana.410400417
Gu M, Cooper JM, Taanman JW, Schapira AH (1998) Mitochondrial DNA transmission of the mitochondrial defect in Parkinson’s disease. Ann Neurol 44:177–186. doi:10.1002/ana.410440207
Luoma P, Melberg A, Rinne JO, Kaukonen JA, Nupponen NN, Chalmers RM, Oldfors A, Rautakorpi I, Peltonen L, Majamaa K, Somer H, Suomalainen A (2004) Parkinsonism, premature menopause, and mitochondrial DNA polymerase gamma mutations: clinical and molecular genetic study. Lancet 364:875–882. doi:10.1016/S0140-6736(04)16983-3
Taanman JW, Schapira AH (2005) Analysis of the trinucleotide CAG repeat from the DNA polymerase gamma gene (POLG) in patients with Parkinson’s disease. Neurosci Lett 376:56–59. doi:10.1016/j.neulet.2004.11.023
Valente EM, Abou-Sleiman PM, Caputo V, Muqit MM, Harvey K, Gispert S, Ali Z, Del Turco D, Bentivoglio AR, Healy DG, Albanese A, Nussbaum R, Gonzalez-Maldonado R, Deller T, Salvi S, Cortelli P, Gilks WP, Latchman DS, Harvey RJ, Dallapiccola B, Auburger G, Wood NW (2004) Hereditary early-onset Parkinson’s disease caused by mutations in PINK1. Science 304:1158–1160. doi:10.1126/science.1096284
Unoki M, Nakamura Y (2001) Growth-suppressive effects of BPOZ and EGR2, two genes involved in the PTEN signaling pathway. Oncogene 20:4457–4465. doi:10.1038/sj.onc.1204608
Kuroda Y, Mitsui T, Kunishige M, Shono M, Akaike M, Azuma H, Matsumoto T (2006) Parkin enhances mitochondrial biogenesis in proliferating cells. Hum Mol Genet 15:883–895. doi:10.1093/hmg/ddl006
Park J, Lee SB, Lee S, Kim Y, Song S, Kim S, Bae E, Kim J, Shong M, Kim JM, Chung J (2006) Mitochondrial dysfunction in Drosophila PINK1 mutants is complemented by parkin. Nature 441:1157–1161. doi:10.1038/nature04788
Clark IE, Dodson MW, Jiang C, Cao JH, Huh JR, Seol JH, Yoo SJ, Hay BA, Guo M (2006) Drosophila pink1 is required for mitochondrial function and interacts genetically with parkin. Nature 441:1162–1166. doi:10.1038/nature04779
Bonifati V, Rizzu P, van Baren MJ, Schaap O, Breedveld GJ, Krieger E, Dekker MC, Squitieri F, Ibanez P, Joosse M, van Dongen JW, Vanacore N, van Swieten JC, Brice A, Meco G, van Duijn CM, Oostra BA, Heutink P (2003) Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science 299:256–259. doi:10.1126/science.1077209
Zhang L, Shimoji M, Thomas B, Moore DJ, Yu SW, Marupudi NI, Torp R, Torgner IA, Ottersen OP, Dawson TM, Dawson VL (2005) Mitochondrial localization of the Parkinson’s disease related protein DJ-1: implications for pathogenesis. Hum Mol Genet 14:2063–2073. doi:10.1093/hmg/ddi211
Xu J, Zhong N, Wang H, Elias JE, Kim CY, Woldman I, Pifl C, Gygi SP, Geula C, Yankner BA (2005) The Parkinson’s disease-associated DJ-1 protein is a transcriptional co-activator that protects against neuronal apoptosis. Hum Mol Genet 14:1231–1241. doi:10.1093/hmg/ddi134
Strauss KM, Martins LM, Plun-Favreau H, Marx FP, Kautzmann S, Berg D, Gasser T, Wszolek Z, Muller T, Bornemann A, Wolburg H, Downward J, Riess O, Schulz JB, Kruger R (2005) Loss of function mutations in the gene encoding Omi/HtrA2 in Parkinson’s disease. Hum Mol Genet 14:2099–2111. doi:10.1093/hmg/ddi215
Martins LM, Morrison A, Klupsch K, Fedele V, Moisoi N, Teismann P, Abuin A, Grau E, Geppert M, Livi GP, Creasy CL, Martin A, Hargreaves I, Heales SJ, Okada H, Brandner S, Schulz JB, Mak T, Downward J (2004) Neuroprotective role of the Reaper-related serine protease HtrA2/Omi revealed by targeted deletion in mice. Mol Cell Biol 24:9848–9862. doi:10.1128/MCB.24.22.9848-9862.2004
Schapira AH, Bezard E, Brotchie J, Calon F, Collingridge GL, Ferger B, Hengerer B, Hirsch E, Jenner P, Le Novere N, Obeso JA, Schwarzschild MA, Spampinato U, Davidai G (2006) Novel pharmacological targets for the treatment of Parkinson’s disease. Nat Rev Drug Discov 5:845–854. doi:10.1038/nrd2087
Schapira AH (2002) The “new” mitochondrial disorders. J Neurol Neurosurg Psychiatry 72:144–149. doi:10.1136/jnnp.72.2.144
Tabrizi SJ, Cleeter MW, Xuereb J, Taanman JW, Cooper JM, Schapira AH (1999) Biochemical abnormalities and excitotoxicity in Huntington’s disease brain. Ann Neurol 45:25–32. doi:10.1002/1531-8249(199901)45:1<25::AID-ART6>3.0.CO;2-E
Mann VM, Cooper JM, Javoy-Agid F, Agid Y, Jenner P, Schapira AH (1990) Mitochondrial function and parental sex effect in Huntington’s disease. Lancet 336:749. doi:10.1016/0140-6736(90)92242-A
Cui L, Jeong H, Borovecki F, Parkhurst CN, Tanese N, Krainc D (2006) Transcriptional repression of PGC-1alpha by mutant huntingtin leads to mitochondrial dysfunction and neurodegeneration. Cell 127:59–69. doi:10.1016/j.cell.2006.09.015
Author information
Authors and Affiliations
Corresponding author
Additional information
Special issue article in honor of Dr. Anna Maria Giuffrida-Stella.
Rights and permissions
About this article
Cite this article
Schapira, A.H.V. Mitochondrial Dysfunction in Neurodegenerative Diseases. Neurochem Res 33, 2502–2509 (2008). https://doi.org/10.1007/s11064-008-9855-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-008-9855-x