Abstract
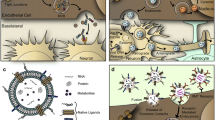
The goal of brain drug targeting technology is the delivery of therapeutics across the blood–brain barrier (BBB), including the human BBB. This is accomplished by re-engineering pharmaceuticals to cross the BBB via specific endogenous transporters localized within the brain capillary endothelium. Certain endogenous peptides, such as insulin or transferrin, undergo receptor-mediated transport (RMT) across the BBB in vivo. In addition, peptidomimetic monoclonal antibodies (MAb) may also cross the BBB via RMT on the endogenous transporters. The MAb may be used as a molecular Trojan horse to ferry across the BBB large molecule pharmaceuticals, including recombinant proteins, antibodies, RNA interference drugs, or non-viral gene medicines. Fusion proteins of the molecular Trojan horse and either neurotrophins or single chain Fv antibodies have been genetically engineered. The fusion proteins retain bi-functional properties, and both bind the BBB receptor, to trigger transport into brain, and bind the cognate receptor inside brain to induce the pharmacologic effect. Trojan horse liposome technology enables the brain targeting of non-viral plasmid DNA. Molecular Trojan horses may be formulated with fusion protein technology, avidin–biotin technology, or Trojan horse liposomes to target to brain virtually any large molecule pharmaceutical.








Similar content being viewed by others
References
W. M. Pardridge. Biochemistry of the human blood–brain barrier. Blood–brain barrier: interface between internal medicine and the brain. Ann. Intern. Med. 105:82–95 (1986).
A. K. Ghose, V. N. Viswanadhan, and J. J. Wendoloski. A knowledge-based approach in designing combinatorial or medicinal chemistry libraries for drug discovery. 1. A qualitative and quantitative characterization of known drug databases. J. Com. Chem. 1:55–68 (1999).
C. A. Lipinski. Drug-like properties and the causes of poor solubility and poor permeability. J. Pharmacol. Toxicol. Methods. 44:235–249 (2000).
N. H. Greig, E. M. Daly, D. J. Sweeney, and S. I. Rapoport. Pharmacokinetics of chlorambucil–tertiary butyl ester, a lipophilic chlorambucil derivative that achieves and maintains high concentrations in brain. Cancer Chemother. Pharmacol. 25:320–325 (1990).
W. M. Pardridge. Brain Drug Targeting: The Future of Brain Drug Development. Cambridge University Press, Cambridge, UK, 2001.
H. Fischer, R. Gottschlich, and A. Seelig. Blood–brain barrier permeation: molecular parameters governing passive diffusion. J. Membr. Biol. 165: 201–211 (1998).
D. J. Hingson and J. M. Diamond. Comparison of nonelectrolyte permeability patterns in several epithelia. J. Membr. Biol. 10:93–135 (1972).
B. E. Cohen and A. D. Bangham. Diffusion of small non-electrolytes across liposome membranes. Nature. 236:173–174 (1972).
W. R. Lieb and W. D. Stein. Non-Stokesian nature of transverse diffusion within human red cell membranes. J. Membr. Biol. 92:111–119 (1986).
R. J. Boado, J. Y. Li, C. Chu, F. Ogoshi, P. Wise, and W. M. Pardridge. Site-directed mutagenesis of cysteine residues of large neutral amino acid transporter LAT1. Biochim. Biophys. Acta 1715:104–110 (2005).
Y. Kanai, H. Segawa, K. Miyamoto, H. Uchino, E. Takeda, and H. Endou. Expression cloning and characterization of a transporter for large neutral amino acids activated by the heavy chain of 4F2 antigen (CD98). J. Biol. Chem. 273:23629–23632 (1998).
R. Pfeiffer, B. Spindler, J. Loffing, P. J. Skelly, C. B. Shoemaker, and F. Verrey. Functional heterodimeric amino acid transporters lacking cysteine residues involved in disulfide bond. FEBS Lett. 439:157–162 (1998).
R. J. Boado, J. Y. Li, and W. M. Pardridge. Site-directed mutagenesis of rabbit LAT1 at amino acids 219 and 234. J. Neurochem. 84:1322–1331 (2003).
R. G. Blasberg, C. Patlak, and J. D. Fenstermacher. Intrathecal chemotherapy: brain tissue profiles after ventriculocisternal perfusion. J. Pharmacol. Exp. Ther. 195:73–83 (1975).
N. P. Christy and R. A. Fishman. Studies of the blood–cerebrospinal fluid barrier to cortisol in the dog. J. Clin. Invest. 40:1997–2006 (1961).
R. B. Aird. A study of intrathecal, cerebrospinal fluid-to-brain exchange. Exp. Neurol. 86:342–358 (1984).
L. K. Fung, M. Shin, B. Tyler, H. Brem, and W. M. Saltzman. Chemotherapeutic drugs released from polymers: distribution of 1,3-bis(2-chloroethyl)-1-nitrosourea in the rat brain. Pharm. Res. 13:671–682 (1996).
C. E. Krewson, M. L. Klarman, and W. M. Saltzman. Distribution of nerve growth factor following direct delivery to brain interstitium. Brain. Res. 680:196–206 (1995).
M. L. Rennels, T. F. Gregory, O. R. Blaumanis, K. Fujimoto, and P. A. Grady. Evidence for a ‘paravascular’ fluid circulation in the mammalian central nervous system, provided by the rapid distribution of tracer protein throughout the brain from the subarachnoid space. Brain Res. 326:47–63 (1985).
M. T. Krauze, R. Saito, C. Noble, J. Bringas, J. Forsayeth, T. R. McKnight, J. Park, and K. S. Bankiewicz. Effects of the perivascular space on convection-enhanced delivery of liposomes in primate putamen. Exp. Neurol. 196:104–111 (2005).
I. Szentistvanyi, C. S. Patlak, R. A. Ellis, and H. F. Cserr. Drainage of interstitial fluid from different regions of rat brain. Am. J. Physiol. 246:F835–844 (1984).
H. Davson, K. Welch, and M.B. Segal. Secretion of cerebrospinal fluid. In The Physiology and Pathophysiology of the Cerebrospinal Fluid. Churchill Livingstone, London, 1987, p. 201.
Y. Ai, W. Markesbery, Z. Zhang, R. Grondin, D. Elseberry, G. A. Gerhardt, and D. M. Gash. Intraputamenal infusion of GDNF in aged rhesus monkeys: distribution and dopaminergic effects. J. Comp. Neurol. 461:250–261 (2003).
T. C. Anand Kumar, G. F. David, A. Sankaranarayanan, V. Puri, and K. R. Sundram. Pharmacokinetics of progesterone after its administration to ovariectomized rhesus monkeys by injection, infusion, or nasal spraying. Proc. Natl. Acad. Sci. U. S. A. 79:4185–4189 (1982).
T. Sakane, M. Akizuki, S. Yamashita, T. Nadai, M. Hashida, and H. Sezaki. The transport of a drug to the cerebrospinal fluid directly from the nasal cavity: the relation to the lipophilicity of the drug. Chem. Pharm. Bull. (Tokyo). 39:2456–2458 (1991).
H. Yamazumi. Infiltration of India ink from subarachnoid space to nasal mucosa along olfactory nerves in rabbits. Nippon Jibi Inkoka Gakkai Kaiho. 92:608–616 (1989).
P. Merkus, H. J. Guchelaar, D. A. Bosch, and F. W. Merkus. Direct access of drugs to the human brain after intranasal drug administration? Neurology. 60:1669–1671 (2003).
W. M. Pardridge. Blood–brain barrier delivery. Drug Discov Today. 12:54–61 (2007).
M. P. van den Berg, P. Merkus, S. G. Romeijn, J. C. Verhoef, and F. W. Merkus. Uptake of melatonin into the cerebrospinal fluid after nasal and intravenous delivery: studies in rats and comparison with a human study. Pharm. Res. 21:799–802 (2004).
W. H. Oldendorf. Brain uptake of radiolabeled amino acids, amines, and hexoses after arterial injection. Am. J. Physiol. 221:1629–1639 (1971).
W. M. Pardridge. Brain metabolism: a perspective from the blood–brain barrier. Physiol. Rev. 63:1481–1535 (1983).
W. M. Pardridge, R. J. Boado, and C. R. Farrell. Brain-type glucose transporter (GLUT-1) is selectively localized to the blood–brain barrier. Studies with quantitative western blotting and in situ hybridization. J. Biol. Chem. 265:18035–18040 (1990).
J. Y. Li, R. J. Boado, and W. M. Pardridge. Cloned blood–brain barrier adenosine transporter is identical to the rat concentrative Na+ nucleoside cotransporter CNT2. J. Cereb. Blood Flow Metab. 21:929–936 (2001).
W. M. Pardridge, T. Yoshikawa, Y. S. Kang, and L. P. Miller. Blood–brain barrier transport and brain metabolism of adenosine and adenosine analogs. J. Pharmacol. Exp. Ther. 268:14–18 (1994).
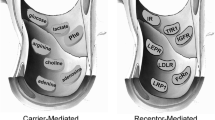
A. Tsuji and I. I. Tamai. Carrier-mediated or specialized transport of drugs across the blood–brain barrier. Adv. Drug. Deliv. Rev. 36:277–290 (1999).
T. Terasaki and K. Hosoya. The blood–brain barrier efflux transporters as a detoxifying system for the brain. Adv. Drug Deliv. Rev. 36:195–209 (1999).
H. Kusuhara and Y. Sugiyama. Efflux transport systems for drugs at the blood–brain barrier and blood–cerebrospinal fluid barrier (Part 1). Drug Discov. Today. 6:150–156 (2001).
T. Terasaki. Development of Brain Efflux Index (BEI) method and its application to the blood–brain barrier efflux transport study. In Introduction to the Blood–Brain Barrier; Methodology, Biology and Pathology, Cambridge University Press, Cambridge, 1998, pp. 24–31.
E. M. Cornford, S. Hyman, M. E. Cornford, and M. J. Caron. Glut1 glucose transporter activity in human brain injury. J. Neurotrauma. 13:523–536 (1996).
K. R. Duffy and W. M. Pardridge. Blood–brain barrier transcytosis of insulin in developing rabbits. Brain Res. 420:32–38 (1987).
J. Holly and C. Perks. The role of insulin-like growth factor binding proteins. Neuroendocrinology. 83:154–160 (2006).
W. M. Pardridge, J. L. Buciak, and P. M. Friden. Selective transport of an anti-transferrin receptor antibody through the blood–brain barrier in vivo. J. Pharmacol. Exp. Ther. 259:66–70 (1991).
Y. Zhang and W. M. Pardridge. Mediated efflux of IgG molecules from brain to blood across the blood–brain barrier. J. Neuroimmunol. 114:168–172 (2001).
H. F. Cserr, D. N. Cooper, P. K. Suri, and C. S. Patlak. Efflux of radiolabeled polyethylene glycols and albumin from rat brain. Am. J. Physiol. 240:F319–F328 (1981).
F. Schlachetzki, C. Zhu, and W. M. Pardridge. Expression of the neonatal Fc receptor (FcRn) at the blood–brain barrier. J. Neurochem. 81:203–206 (2002).
D. Triguero, J. Buciak, and W. M. Pardridge. Capillary depletion method for quantification of blood–brain barrier transport of circulating peptides and plasma proteins. J. Neurochem. 54:1882–1888 (1990).
H. J. Lee, B. Engelhardt, J. Lesley, U. Bickel, and W. M. Pardridge. Targeting rat anti-mouse transferrin receptor monoclonal antibodies through blood–brain barrier in mouse. J. Pharmacol. Exp. Ther. 292:1048–1052 (2000).
W. M. Pardridge, Y. S. Kang, J. L. Buciak, and J. Yang. Human insulin receptor monoclonal antibody undergoes high affinity binding to human brain capillaries in vitro and rapid transcytosis through the blood–brain barrier in vivo in the primate. Pharm. Res. 12:807–816 (1995).
D. Wu, J. Yang, and W. M. Pardridge. Drug targeting of a peptide radiopharmaceutical through the primate blood–brain barrier in vivo with a monoclonal antibody to the human insulin receptor. J. Clin. Invest. 100:1804–1812 (1997).
T. S. Salahuddin, B. B. Johansson, H. Kalimo, and Y. Olsson. Structural changes in the rat brain after carotid infusions of hyperosmolar solutions. An electron microscopic study. Acta Neuropathol. (Berlin). 77:5–13 (1988).
A. S. Lossinsky, A. W. Vorbrodt, and H. M. Wisniewski. Scanning and transmission electron microscopic studies of microvascular pathology in the osmotically impaired blood–brain barrier. J. Neurocytol. 24:795–806 (1995).
E. A. Neuwelt and S. I. Rapoport. Modification of the blood–brain barrier in the chemotherapy of malignant brain tumors. Fed. Proc. 43:214–219 (1984).
L. I. Larsson, J. Fahrenkrug, O. Schaffalitzky De Muckadell, F. Sundler, R. Hakanson, and J. R. Rehfeld. Localization of vasoactive intestinal polypeptide (VIP) to central and peripheral neurons. Proc. Natl. Acad. Sci. U. S. A. 73:3197–3200 (1976).
J. McCulloch and L. Edvinsson. Cerebral circulatory and metabolic effects of vasoactive intestinal polypeptide. Am. J. Physiol. 238:H449–H456 (1980).
D. Wu and W. M. Pardridge. Central nervous system pharmacologic effect in conscious rats after intravenous injection of a biotinylated vasoactive intestinal peptide analog coupled to a blood–brain barrier drug delivery system. J. Pharmacol. Exp. Ther. 279:77–83 (1996).
D. Wu and W. M. Pardridge. Neuroprotection with noninvasive neurotrophin delivery to the brain. Proc. Natl. Acad. Sci. U. S. A. 96:254–259 (1999).
T. Sakane and W. M. Pardridge. Carboxyl-directed pegylation of brain-derived neurotrophic factor markedly reduces systemic clearance with minimal loss of biologic activity. Pharm. Res. 14:1085–1091 (1997).
Y. Zhang and W. M. Pardridge. Conjugation of brain-derived neurotrophic factor to a blood–brain barrier drug targeting system enables neuroprotection in regional brain ischemia following intravenous injection of the neurotrophin. Brain Res. 889:49–56 (2001).
Y. Zhang and W. M. Pardridge. Neuroprotection in transient focal brain ischemia after delayed intravenous administration of brain-derived neurotrophic factor conjugated to a blood–brain barrier drug targeting system. Stroke. 32:1378–1384 (2001).
B. W. Song, H. V. Vinters, D. Wu, and W. M. Pardridge. Enhanced neuroprotective effects of basic fibroblast growth factor in regional brain ischemia after conjugation to a blood–brain barrier delivery vector. J. Pharmacol. Exp. Ther. 301:605–610 (2002).
L. Belayev, R. Busto, W. Zhao, and M. D. Ginsberg. Quantitative evaluation of blood–brain barrier permeability following middle cerebral artery occlusion in rats. Brain Res. 739:88–96 (1996).
A. Kurihara and W. M. Pardridge. Imaging brain tumors by targeting peptide radiopharmaceuticals through the blood–brain barrier. Cancer Res. 59:6159–6163 (1999).
A. Kurihara, Y. Deguchi, and W. M. Pardridge. Epidermal growth factor radiopharmaceuticals: 111In chelation, conjugation to a blood–brain barrier delivery vector via a biotin-polyethylene linker, pharmacokinetics, and in vivo imaging of experimental brain tumors. Bioconjug. Chem. 10:502–511 (1999).
H. J. Lee, Y. Zhang, C. Zhu, K. Duff, and W. M. Pardridge. Imaging brain amyloid of Alzheimer disease in vivo in transgenic mice with an Abeta peptide radiopharmaceutical. J. Cereb. Blood Flow Metab. 22:223–231 (2002).
Y. Saito, J. Buciak, J. Yang, and W. M. Pardridge. Vector-mediated delivery of 125I-labeled beta-amyloid peptide A beta 1–40 through the blood–brain barrier and binding to Alzheimer disease amyloid of the A beta 1–40/vector complex. Proc. Natl. Acad. Sci. U. S. A. 92:10227–10231 (1995).
T. Suzuki, D. Wu, F. Schlachetzki, J. Y. Li, R. J. Boado, and W. M. Pardridge. Imaging endogenous gene expression in brain cancer in vivo with 111In-peptide nucleic acid antisense radiopharmaceuticals and brain drug-targeting technology. J. Nucl. Med. 45:1766–1775 (2004).
T. Suzuki, Y. Zhang, Y. F. Zhang, F. Schlachetzki, and W. M. Pardridge. Imaging gene expression in regional brain ischemia in vivo with a targeted [111in]-antisense radiopharmaceutical. Mol. Imaging 3:356–363 (2004).
W. M. Pardridge, R. J. Boado, and Y. S. Kang. Vector-mediated delivery of a polyamide (“peptide”) nucleic acid analogue through the blood–brain barrier in vivo. Proc. Natl. Acad. Sci. U. S. A. 92:5592–5596 (1995).
Y. Zhang and W. M. Pardridge. Delivery of beta-galactosidase to mouse brain via the blood–brain barrier transferrin receptor. J. Pharmacol. Exp. Ther. 313:1075–1081 (2005).
M. J. Coloma, H. J. Lee, A. Kurihara, E. M. Landaw, R. J. Boado, S. L. Morrison, and W. M. Pardridge. Transport across the primate blood–brain barrier of a genetically engineered chimeric monoclonal antibody to the human insulin receptor. Pharm. Res. 17:266–274 (2000).
R. J. Boado, Y. Zhang, and W. M. Pardridge. Humanization of anti-human insulin receptor antibody for drug targeting across the human blood–brain barrier. Biotechnol. Bioeng. 96:381–391 (2007).
R. J. Boado, Y. Zhang, and W. M. Pardridge. Genetic engineering, expression, and activity of a fusion protein of a human neurotrophin and a molecular Trojan horse for delivery across the human blood–brain barrier. Biotechnol. Bioeng. in press (2007).
R. J. Boado, Y. Zhang, C. F. Xia, and W. M. Pardridge. Fusion antibody for Alzheimer’s disease with bidirectional transport across the blood–brain barrier and abeta fibril disaggregation. Bioconjug Chem. 18:447–455 (2007).
J. Norman, W. Denham, D. Denham, J. Yang, G. Carter, A. Abouhamze, C. L. Tannahill, S. L. MacKay, and L. L. Moldawer. Liposome-mediated, nonviral gene transfer induces a systemic inflammatory response which can exacerbate pre-existing inflammation. Gene Ther. 7:1425–1430 (2000).
D. Simberg, S. Weisman, Y. Talmon, A. Faerman, T. Shoshani, and Y. Barenholz. The role of organ vascularization and lipoplex-serum initial contact in intravenous murine lipofection. J. Biol. Chem. 278:39858–39865 (2003).
N. Shi and W. M. Pardridge. Noninvasive gene targeting to the brain. Proc. Natl. Acad. Sci. U. S. A. 97:7567–7572 (2000).
Y. Zhang, F. Schlachetzki, and W. M. Pardridge. Global non-viral gene transfer to the primate brain following intravenous administration. Mol. Ther. 7:11–18 (2003).
N. Shi, Y. Zhang, C. Zhu, R. J. Boado, and W. M. Pardridge. Brain-specific expression of an exogenous gene after i.v. administration. Proc. Natl. Acad. Sci. U. S. A. 98:12754–12759 (2001).
N. Shi, R. J. Boado, and W. M. Pardridge. Receptor-mediated gene targeting to tissues in vivo following intravenous administration of pegylated immunoliposomes. Pharm. Res. 18:1091–1095 (2001).
Y. Zhang, F. Schlachetzki, J. Y. Li, R. J. Boado, and W. M. Pardridge. Organ-specific gene expression in the rhesus monkey eye following intravenous non-viral gene transfer. Mol. Vis. 9:465–472 (2003).
D. J. Zack, J. Bennett, Y. Wang, C. Davenport, B. Klaunberg, J. Gearhart, and J. Nathans. Unusual topography of bovine rhodopsin promoter-lacZ fusion gene expression in transgenic mouse retinas. Neuron. 6:187–199 (1991).
C. Zhu, Y. Zhang, Y. F. Zhang, J. Yi Li, R. J. Boado, and W. M. Pardridge. Organ-specific expression of the lacZ gene controlled by the opsin promoter after intravenous gene administration in adult mice. J. Gene Med. 6:906–912 (2004).
Y. Zhang, F. Schlachetzki, Y. F. Zhang, R. J. Boado, and W. M. Pardridge. Normalization of striatal tyrosine hydroxylase and reversal of motor impairment in experimental parkinsonism with intravenous nonviral gene therapy and a brain-specific promoter. Hum. Gene Ther. 15:339–350 (2004).
Y. Zhang, C. Zhu, and W. M. Pardridge. Antisense gene therapy of brain cancer with an artificial virus gene delivery system. Mol. Ther. 6:67–72 (2002).
Y. Zhang, Y. F. Zhang, J. Bryant, A. Charles, R. J. Boado, and W. M. Pardridge. Intravenous RNA interference gene therapy targeting the human epidermal growth factor receptor prolongs survival in intracranial brain cancer. Clin. Cancer Res. 10:3667–3677 (2004).
W. M. Pardridge. Recent advances in blood–brain barrier transport. Annu. Rev. Pharmacol. Toxicol. 28:25–39 (1988).
Y. F. Zhang, R. J. Boado, and W. M. Pardridge. Absence of toxicity of chronic weekly intravenous gene therapy with pegylated immunoliposomes. Pharm. Res. 20:1779–1785 (2003).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pardridge, W.M. Drug Targeting to the Brain. Pharm Res 24, 1733–1744 (2007). https://doi.org/10.1007/s11095-007-9324-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-007-9324-2